Colloids and it’s Classification
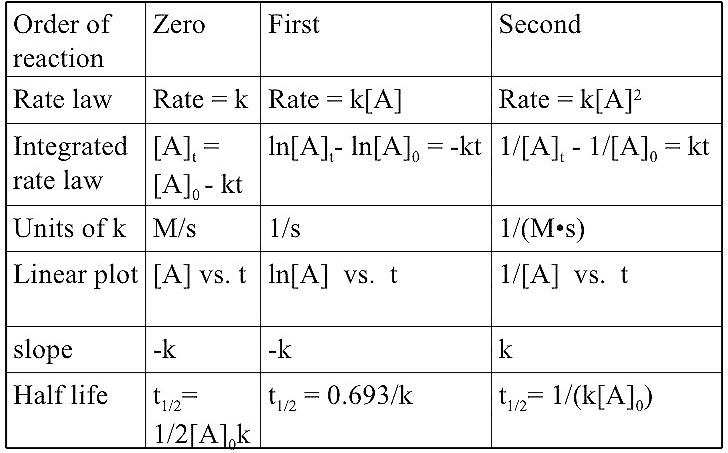
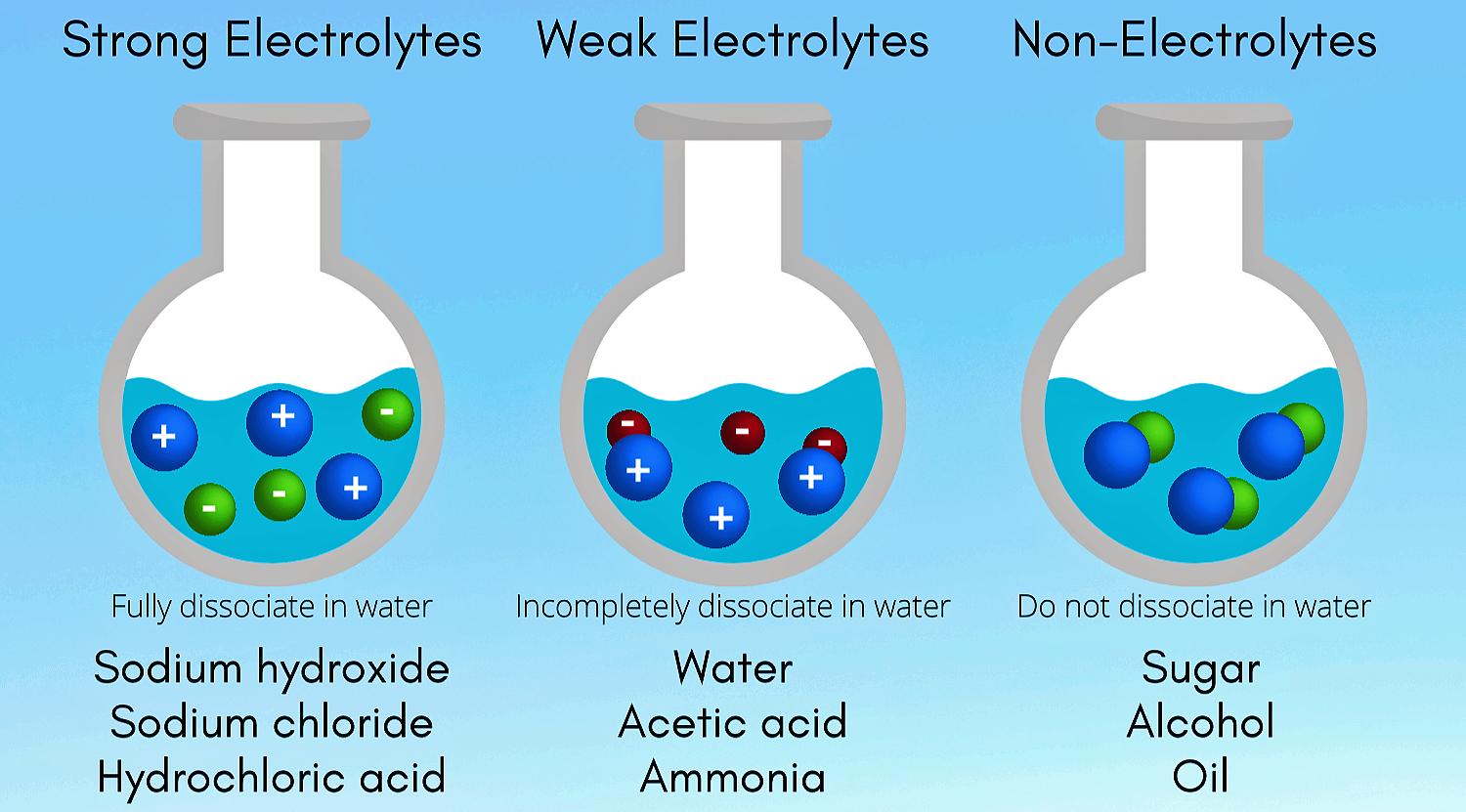
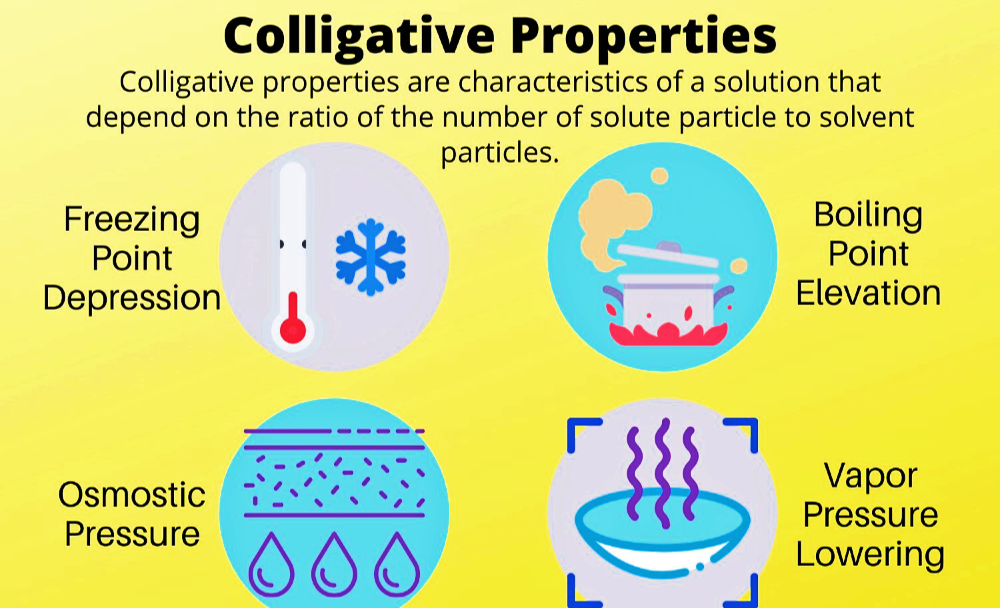
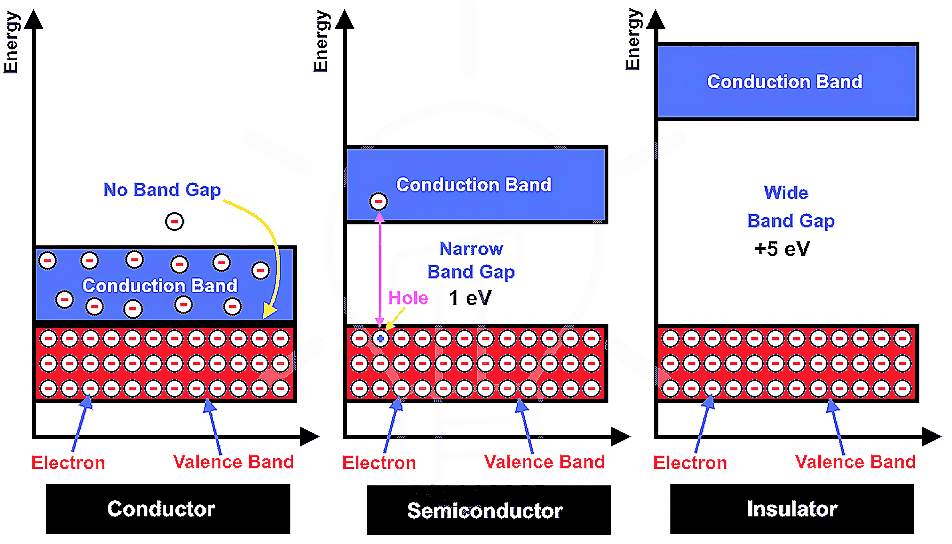
Colloids :- A Colloid is a heterogeneous system in which one substance is dispersed ( dispersed phase ) as very fine particles in another substance called dispersion medium. Colloidal particles have and enormous surface area per unit mass as a result of their small size. Consider a cube with 1 cm side. It has a…