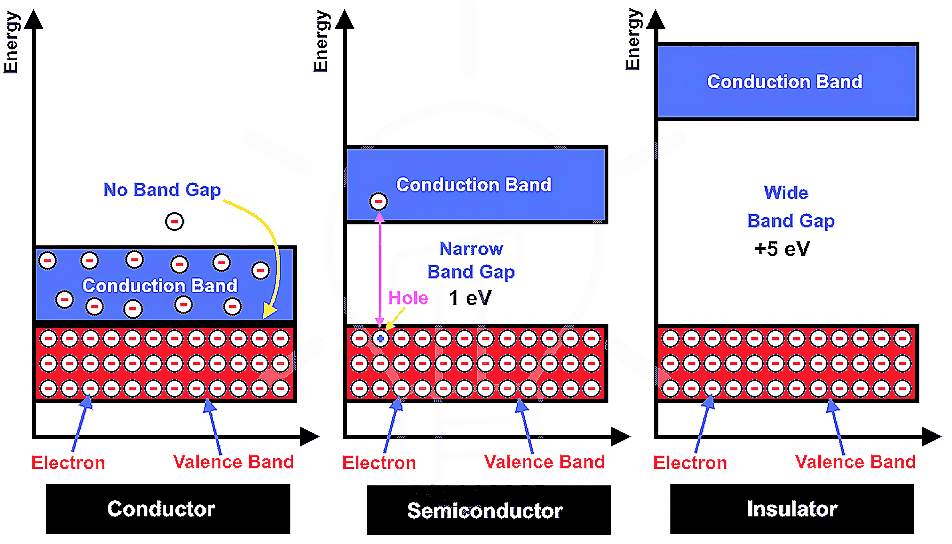
Conductors:-
The solids with conductivities ranging between 10⁴ to 10⁷ ohm⁻¹ m⁻¹ are called conductors.
Examples. – Metals like Iron, Copper, Sodium etc.

- A conductor may conduct electricity in solid as well as molten state. The conductivity of metals depends upon the number of valence electrons available per atom.
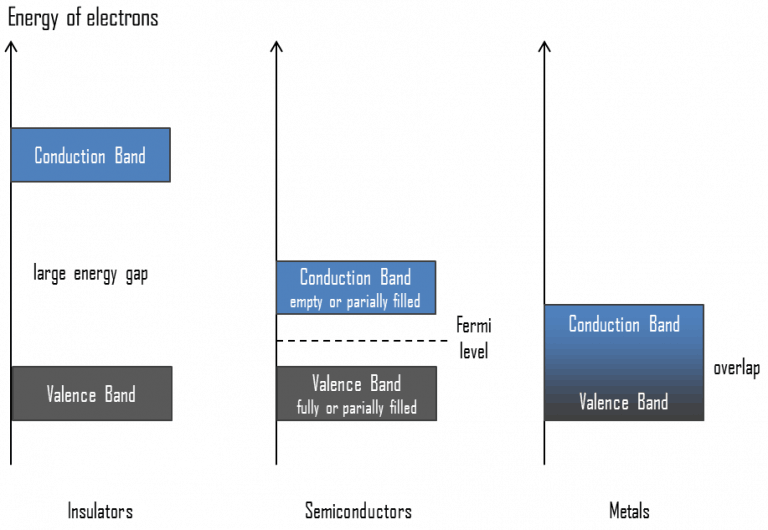
- The atomic orbitals of metal atoms form molecular orbitals which are so close in energy to each other as to form a band. If this band is partially filled or It overlaps with a higher energy unoccupied conduction band, then electrons can flow easily under an applied electric field and the metal shows conductivity.
Insulators :-
- These are the solids with very low coductivities ranging between 10⁻²⁰ to 10⁻¹⁰ ohm⁻¹ m⁻¹·
- If the gap between filled valence band and the next higher unoccupied ( conduction band ) is large, electrons cannot jump to it and such a substance has very small conductivity and it behaves as an insulator.
- Examples – Generally non metals are Insulators.

Semiconductors :-
These are the solids with conductivities in the intermediate range from 10⁻⁶ to 10⁴ ohm⁻¹ m⁻¹·
- In case of semiconductors, the gap between valence band and conduction band is small, therefore some electrons may jump to conduction and show some conductivity.
- Electrical conductivity of semiconductors increases with rise in temperature, since more electrons can jump to the conduction band.
Examples. – Silicon and Germenium shows this behaviour called intrinsic semiconductor.
- The conductivity is increased by adding and appropriate amount of suitable impurity, this process is called doping.
Types of Semiconductors :-
It can be of two types. –
1. n – type semiconductor.
2. P – type Semiconductor.
n – type Semiconductor :-

Silicon ( Si )and Germenium ( Ge ) belong to group 14 of the periodic table and have four valence electrons each. when doped with a group 15 elements like Phosphorus ( P ) or Arsenic ( As ) which contains 5 valence electrons, they occupy some of the lettice sites in Silicon or Germenium crystal. 4 out of 5 electrons are used in the formation of covalent bonds with the four neighbouring Silicon atoms.
The fifth electron is extra and becomes delocated in increase the conductivity of doped Silicon.
Here the increase in conductivity is due to the negatively charged electron, hence conductor is called n – type semiconductor.
P -type semiconductor :-

Silicon or Germenium can doped with a group 13 elements like Boron, Aluminium or Gallium which contains only three valence electrons.
The place where the fourth electron is missing is called hole or electron vacancy. If the electron hole has moved in the direction opposite to that of the electron that filled it.
Under the influence of electronic field, electrons would move towards the positively charged plate through electronic holes, but it would appear as if electron holes are positively charged and are moving towards negatively charged plate. This type of Semiconductors are called P- type Semiconductors.