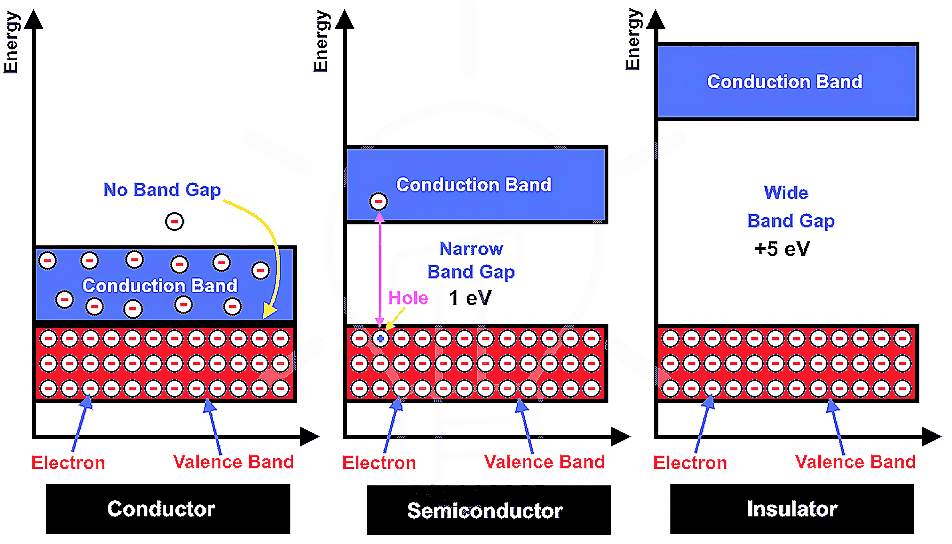
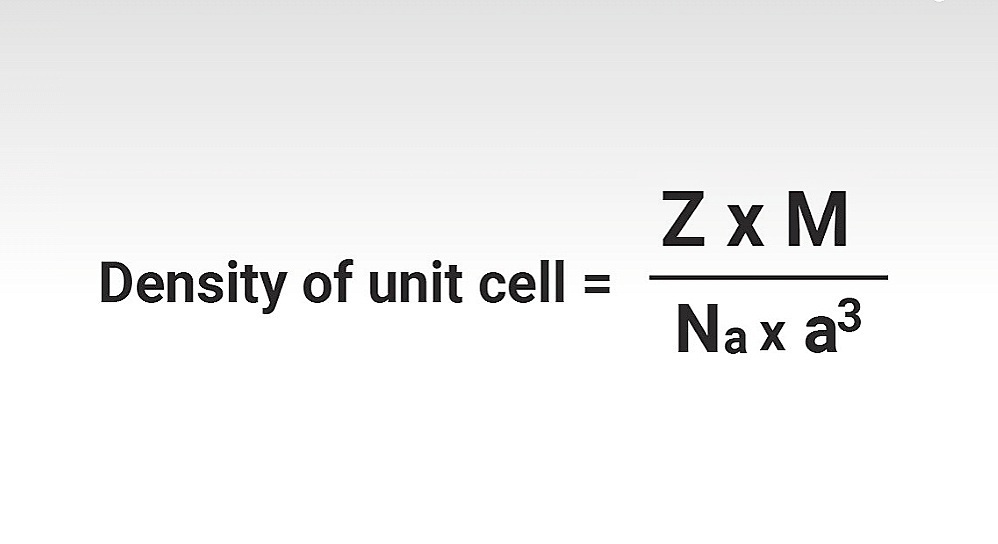Magnetic properties of Crystals : Paramagnetic, Diamagnetic, Ferromagnetic, Antiferromagnetic and Ferrimagnetic substances
Magnetic properties of Crystals :- Each electron in an atom behaves like a magnet. It’s magnetic moment originates from two types of motions are orbital motion around the nucleus and spin around its own axis. Magnitude of magnetic moment is very small and is measured in the unit called Bohr magneton μB. It is equal…