Solid state :-
- They have definite mass, volume and shape.
- Solid state can still oscillate about their mean positions.
- Intermolecular forces are strong because their thermal energy is low and intermolecular forces bring them so close that they cling to one another.
- Their constituent particles ( atoms, molecules or ions ) have fixed positions.
- They are incompressible and rigid.
Types of Solid :-
On the basis of the nature of order present in the arrangement of their constituent particles, Solid or solid state can be classified as Crystalline and Amorphous.
Crystalline Solid :-
A crystalline solid state usually consists of a large number of small crystals, each of them having a definite characteristic geometrical shape.
- It has long range order which means that there is a regular pattern of arrangement of particles which repeats itself periodically over the entire crystal.
- Crystalline solid state have a sharp melting point.
- Crystalline solid states are anisotropic in nature, that is some of their physical properties like electrical resistance or refractive index show different values when measured along different directions in the same crystals.
- When cut with a sharp edged tool, they split into two pieces and the newly generated surfaces are plain and smooth.
- They have a definite and characteristic heat of fusion.
- They are true solids.
Example. Sodium chloride and quartz.
Amorphous solid :-
An amorphous solid state consists of particles of irregular shape.
- The arrangement of constituent particles ( atoms, molecules or ions ) in such a solid has only short range order. In such an arrangement, a regular and periodically repeating pattern is observed over short distances only. Such portions are scattered and in between the arrangement is disordered.
- The structure of amorphous solid is similar to that of liquid.
- Amorphous solid States soften over a range of temperature and can be moulded and blown into various shapes. On heating they become crystalline at some temperature.
- Like liquids, amorphus solid States have a tendency to flow through very slowly. Therefore, sometimes these are called pseodo solids or supper cooled liquids.
- Amorphous solids are isotropic in nature. It is because there is no long range order in them and arrangement is irregular along all the directions.
- When cut with a sharp edged tool, they cut into two pieces with irregular surfaces.
- They do not have definite heat of fusion.
- They are pseudo solids or super cooled liquids only short range order.
Example. Glass, rubber, plastics and silicon.
Crystalline solid can be classified on the basis of nature of intermolecular forces operating in them into four categories.
( 1 ) Molecular solids
( 2 ) Ionic solids
( 3 ) Metallic solids
( 4 ) covalent solids.
Molecular solids :-
Molecules are the constituent particles of molecular solids.
These are further sub divided into following categories –
( a ) Non polar molecular solids :-
The molecules formed by non polar covalent bonds are called non polar solids.
Examples. – H₂ , Cl₂ , I₂ etc.
Characteristics :-
- In these solids, the atoms or molecules are held by weak dispersion forces or London forces.
- The solids are soft and non- conductors of electricity.
- They have low melting points and are usually in liquid or gaseous state at room temperature and pressure.
- ( b ) Polar molecular solids :- The molecules formed by polar covalent bonds are called polar molecular solids.
Examples. solid SO₂ , solid NH₃ , Hcl etc.
Characteristics :-
- In these solids, the molecules are held togather by relatively stronger dipole – dipole interactions.
- These solids are soft and non-conductors of electricity.
- Their melting points are higher than those of non-polar molecular solids.
- Most of these are gases or liquids under room temperature and pressure.
( c ) Hydrogen bonded molecular solids:-
The molecules of such solids contain polar covalent bonds between H and F, O or N atoms.
Strong hydrogen bonding binds molecules of such solids like H₂O ( ice ).
Characteristics :-
- They are non conductors of electricity.
- Generally they are volatile liquids or soft solids under room temperature and pressure.
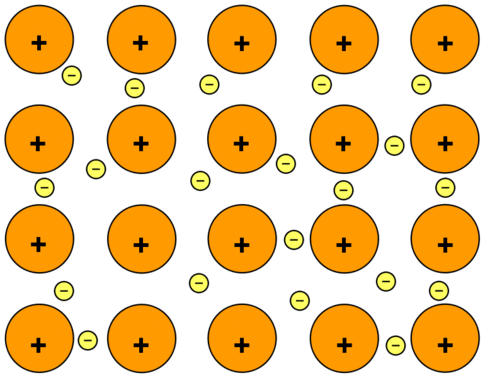
Ionic Solids :-
Ions are the constituent particles of ionic solids. Such solids are formed by three dimensional arrangements of cations (+) and anions (-) bound by strong electrostatic forces.
Example. NaCl, LiF

Characteristics :-
- These solids are hard and brittle in nature.
- They have high melting and boiling points.
- Since the ions are not free to move about, they are electrical insulators in the solid state when dissolved in water, the ions become free to move about and they conduct electricity.
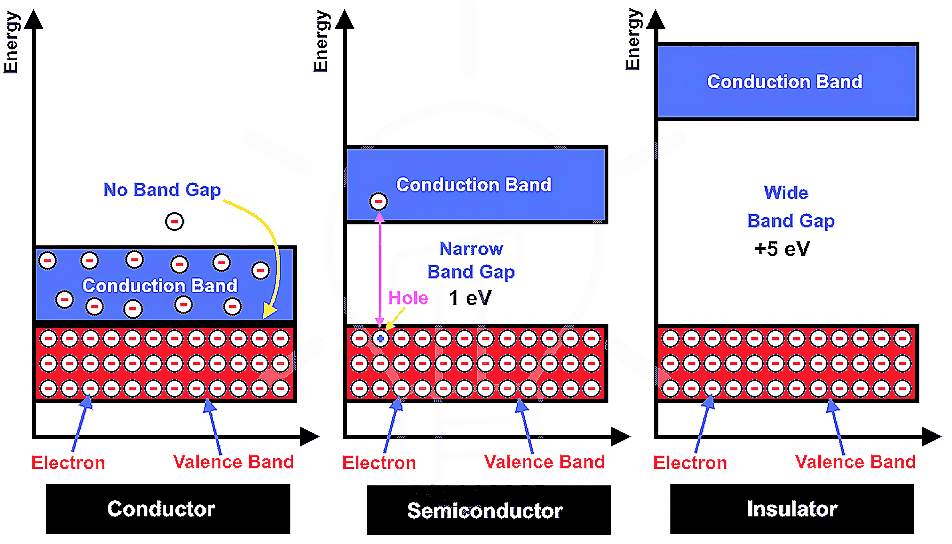
Metallic Solids :-
Metallic Crystal ( Metals ) are orderly collection of positive ions surrounded by and held togather by the electron cloud ( sea of free electrons ). These electrons are evenly spread throughout the Crystal.
Example. – Iron, Copper, Alluminium, Zinc, Magnesium etc.
Characteristics :-
- They are conductor of heat and electricity due to presence of mobile electrons in the Metallic Crystal.
- They are highly malleable and ductile.
- Another important characteristic of metal is their lustre and colour in certain cases.
Covalent or Network Solids :-
A wide veriety of crystalline solids of non-metals result from the formation of covalent bonds between adjacent atoms throughout the crystal. They are also called gaint molecules.
Example. SiO₂ ( quartz ), SiC, C ( Diamond ), C ( Graphite ) etc.

Graphite ( C ) :- It is soft and a conductor of electricity its exceptional properties are due to its typical structure. Carbon atoms are arranged in different laysrs and each atom is covalently bonded to three of its neighbouring atoms in the same layer. The fourth valence electron of each atom is present between different layers and is free to move about, these free electrons make graphite a good conductor of electricity.
Different layers can slide one over the other, this makes graphite a soft solid and a good solid lubricant.
Charactristics :-
- Covalent bonds are strong and directional in nature therefore atoms are held very strongly at their positions, such solids are very hard and brittle.
- They have extremely high melting points and may even decomposed before melting.
- They are insulators and don’t conduct electricity.