Crystal Lattice :-
If the three dimensional arrangement of constituent particles like atoms, molecules or ions in a crystal is represented diagrammatically, in which each particle is depicted as a point, the arrangement is called crystal lattice.
- Each point in a crystal lattice is called lattice point or lattice site. There are mainly three kinds of lattice points, that is, at the corners, at the face centres and within the unit cell.
- In a Crystal lattice , lattice points are joined by straight lines to bring out the geometry of the lattice.
- Bravais Lattices :-
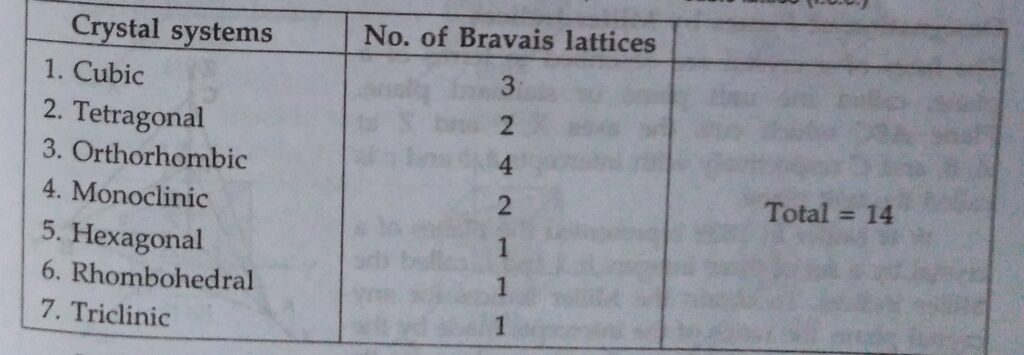
In 1848, Auguste Bravais showed with the help of geometrical calculations that there are 14 possible three dimensional Crystal lattices, these are called Bravais lattices.

Crystal systems :-
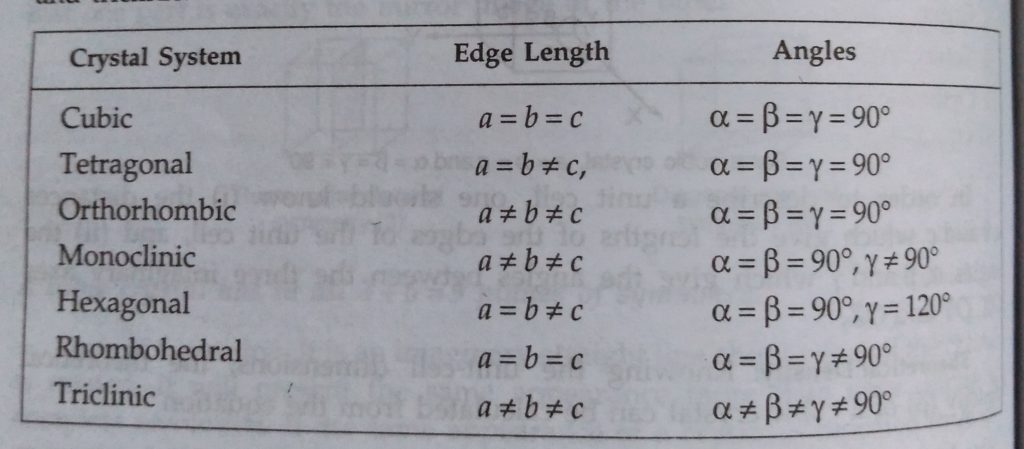
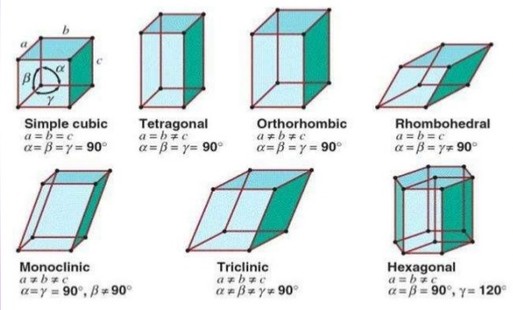
It can be shown from geometrical considrations that theoratically, there can be 32 different combinations of elements of symmetry of a crystal. There are called 32 point groups or 32 systems. However, some of the systems have been grouped so that there are only 7 basic crystal systems, – cubic, tetragonal, orthorhombic, monoclinic, hexagonal, rhombohedral or trigonal or triclinic.
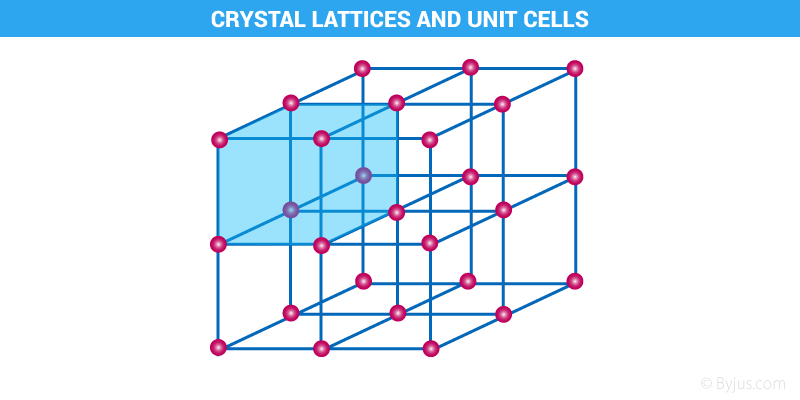
Unit Cell :-
A unit cell is the smallest sample that represents the picture of the entire crystal lattice . A unit cell of a crystal possesses all the structural properties of the given crystal.

Its dimensions along the three edges :- a, b and c . These edges may or may not be mutually perpendicular.
Angles between the edges α, β and y · Thus, a unit cell is characterised by six parameters – a, b, c, α, β and y.
Types of Unit Cells :-
( a ) Primitive Unit Cells.
( b ) Centred Unit Cells.
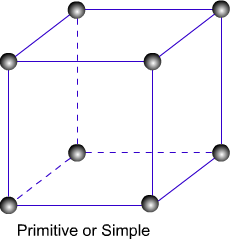
Primitive Unit Cells :-
When constituent particles are present only on the corner positions of a unit cell, it is called primitive unit cell.
Centred Unit Cells :-
They are of three types :-
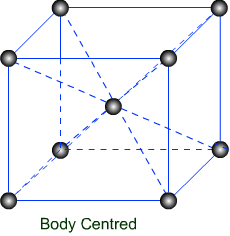
( 1 ) Body Centred Unit Cell :- If the particles are present at the centres of the cell in addition to the corners, then body centred unit is produced.
( 2 ) Face Centred Unit Cell :- If the particles are located at the centre of each face in addition to the corners, then face centred unit cell is produced.

( 3 ) End face Centred Unit Cell :- If the particles are located at the centre of end face in addition to the corners, then end face centred unit is produced.

Unit Cell content ( Z ) :-
( 1 ) primitive or simple unit cell :- A simple cubic unit cell has eight atoms at eight corners and each atom is shared by eight unit cells.
Number of atoms in a simple cubic cell ( Z ) = no. of corner atom x contribution to one unit cell.
or Z = 8 x 1/8
or Z = 1
( 2 ) Body Centred Cubic unit cell :- It has eight atoms at eight corners and one atom at the body centre which is not shared by other cells.
Thus, for body centred cubic unit cell.
Z = 8 x 1/8 +1 = 2
( 3 ) Face Centred Cubic unit cell :- It has eight atoms at the eight corners and six atoms at the six faces. The corner atoms are shared by eight unit cells, while the atom at the face is shared by two unit cells.
Thus, for a face centred cubic unit cell,
Z = 8 x 1/8 + 6 x 1/2 = 4