A solid consists of an aggregation of large number of small crystals, these small crystals have defects in them which are called defects in crystals. The defects in crystals are basically irregularities in the arrangement of constituent particles.
Types of defects in crystals :-
The defects in crystals are of mainly two types:-
- Point defects:- They are the irregularities or deviations from ideal arrangement around a point or an atom in a crystalline substance.
- Line defects:- They are the irregularities or deviations from ideal arrangement in entire rows of lattice points. These irregularities are called crystal defects.
- Types of point defects :- It is of three types –
( a ) Stoichiometry defects.
( b ) Impurity defects.
( c ) Non-stoichiometry defects.
(a) Stoichiometric defects :-
The point defects that do not disturb the stoichiometry of the solid, they are also called intrinsic or thermodynamic defects.
Stoichiometric defects can be of two types-
(i) Vacancy defects
(ii) Interstitial defects
(i) Vacancy defects :-
when some of the Lattice sites are vacant the Crystal is said to have vacancy defect, this results in decrease in density of the substance. This defect can also develop when a substance is heated.
- Schottky defects :-

Schottky defect is basically a vacancy defect in ionic solids, In order to maintain electrical neutrality, the number of missing cations and anions are equal.
Schottky defect also decreases the density of the substance.
Example. – NaCl, KCl, CsCl and AgBr are shows Schottky defects.
AgBr shows both frenkel as well as Schottky defects.
(ii). Interstitial defects :-
when some constituent particles ( atoms or molecules ) occupy an interstitial site, The Crystal is said to have interstitial defect. Interstitial defect increases the density of the substance. Frenkel defect :-

Fig. 2. Frenkel defect in Crystal.
Frenkel defect is shown by ionic solids, the smaller and usually cation is dislocated from its normal site to an interstitial site.
It creates a vacancy defect at its original site and an interstitial defect at its new location. Frankel defect is also called this location defect.It does not change the density of the solid. It is shown by ionic substance in which there is a large difference in the size of ions.
Example. – Zns, AgCl, AgBr, AgI etc. Here small size of Zn²⁺ and Ag⁺ ions.
(b) Impurity defects :-
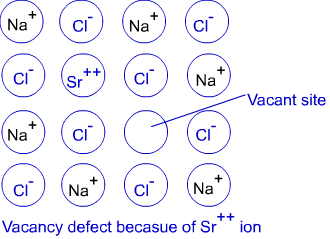
If molten NaCl containing a little amount of of SrCl₂ is crystallised, Some of the sites of Na⁺ ions are occupied by a Sr²⁺ . Each Sr²⁺ replaces two Na⁺ ions· It occupies the site of on iron and the other side remains vacant. The cation vecancies thus produced are equal in number to that of Sr²⁺ ions·
Cdcl₂ and AgCl solid solution is also another similar example of impurity defects.
(c) Non-stoichiometric defects :-
The defects that disturb the stoichiometry of the solid is called Non-stoichiometric defects.
A large number of Non-stoichiometric inorganic solids are known which contain the constituent elements in Non-stoichiometric ratio due to defects in their crystal structure.
- These defects are of two types. –
- Metal excess defect.
- Metal deficiency defect.
- Metal excess defect :-
Metal excess defect due to anionic vacancies :-
In this case negative ions may be missing from their lattice sites leaving holes in which the electrons remain entropped to maintain the electrical neutrality.
This type of defect is observed in those crystals which are likely to form schottky defects.
Example. – Alkali metal halides like NaCl, LiCl, KCl show this type of defect.

- Metal excess defect due to the present of extra cations at interstitial site :-
In this case there are extra cations occupying interstitial sites and the electrons in another interstitial sites to maintain electrical neutrality.
Example. – ZnO
Metal excess defect may be visualised as the loss of non- metal atoms which leave their electrons behind. The excess metal ions occupy interstitial positions.
- Metal deficiency defect :-
In this a cation is missing from its lattice site.
To maintain electrical neutrality, one of the nearest metal ion acquires to positive charge.
This type of defect occurs in compounds like NiO, FeO, FeS etc.