Colloids :-
A Colloid is a heterogeneous system in which one substance is dispersed ( dispersed phase ) as very fine particles in another substance called dispersion medium.
Colloidal particles have and enormous surface area per unit mass as a result of their small size. Consider a cube with 1 cm side. It has a total surface area of 6 cm²
Classification of Colloids :-
Colloids are classified on the basis of the following criteria :
( 1 ) physical state of dispersed phase and dispersion medium.
( 2 ) nature of interaction between dispersed phase and dispersion medium.
( 3 ) Type of particles of the dispersed phase.
Classification based on physical state of dispersed phase and dispersion medium :-
Depending upon whether the dispersed phase and the dispersion medium are solids, liquids or gases, eight types of Colloidal systems are possible.

Out of the various types of colloids given in tables, the most common are sols ( solids in liquids ), gels ( liquids in solids ) and emulsions ( liquids in liquids ).
Classification based on nature of interaction between dispersed phase and dispersion medium :-
Depending upon the nature of interaction between the dispersed phase and the dispersion medium, Colloidal sols are divided into two categories namely, lyophilic ( solvent attracting ) and lyophobic ( solvent repelling ).
Lyophilic Colloids :-
The word lyophic means liquid loving. Colloidal sols directly formed by mixing substances like gum, gelatine, starch, rubber etc. , with a suitable liquid ( the dispersion medium ) are called lyophilic sols.
- If the dispersion medium is separated from the dispersed phase ( say by evaporation ), the sol can be reconstituted by simply remixing with the dispersion medium. These sols are also called reversible sols.
Lyophobic Colloids :-
The word lyophobic means liquid hating. Substances like metals and their sulphides etc. when simply mixed with the dispersion medium do not form the colloidal sol. Their Colloidal sols can be prepared only by a special methods. Such sols are called lyophobic sols.
- These sols are readily precipitated ( or coagulated ) on the addition of small amounts of electrolytes, by heating or by shaking and hence, are not stable. these sols are also called Irreversible sols.
- Lyophobic sols need a stabilising agents for their preservation.
Classification based on type of particles of the dispersed phase, Multimolecular, Macromolecular and associated Colloids :-
Depending upon the type of particles of the dispersed Phase, Colloids are classified as multimolecular, macromolecular and associated colloids.
Multimolecular colloids :-
On dissolution, a large number of atoms or smaller molecules of a substance aggregate together to form a species having size in the colloidal range ( 1 to 1000 nm ). The species thus formed are called multimolecular colloids.
Example, of gold sol may contain particles of various sizes having many atoms.
Macromolecular Colloids :-
Macromolecules in suitable solvents form solutions in which the size of the macromolecules may be in the Colloidal range.
Such systems are called macromolecular colloids. They are quite stable and reassemble true solutions in many respects.
Examples of naturally occurring macromolecules are starch, cellulose, proteins and enzymes, and those of man-made macromolecules are polythene, nylon, polystyrene, synthetic rubber etc.
Associated Colloids ( micelles ) :-
There are some substances which at low concentrations behave as normal strong electrolytes, but at higher concentrations exihibit Colloidal behaviour due to the formation of aggregates. The aggregated particles thus formed are called micelles. These are also known as associated colloids.
- The formation of micelles takes place only above a particular temperature called Kraft temperature and above a particular concentration called critical micelle concentration.
These Colloids have both lyophobic and lyophilic parts. Micelles may contain as many as 100 molecules or more.
Mechanism of Micelle formation :-
Let us take the example of soap solutions, soap is sodium or potassium salt of higher fatty acid and may be represented as RCOO⁻Na⁺ ( example – Sodium stearate CH₃(CH₂)₁₆ COO⁻NA⁺, which is a major component of many bar soaps ).
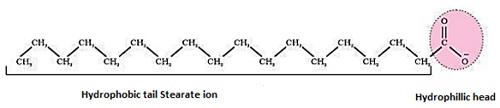
- When dissolved in water, it dissociated into RCOO⁻ and Na⁺ ions. The RCOO⁻ ions, however consists of two parts – a long hydrocarbon chain R ( also called non polar tail ) which is hydrophobic ( water repelling) and polar group COO⁻ (also called polar ionic head ) which is hydrophilic ( water loving ).
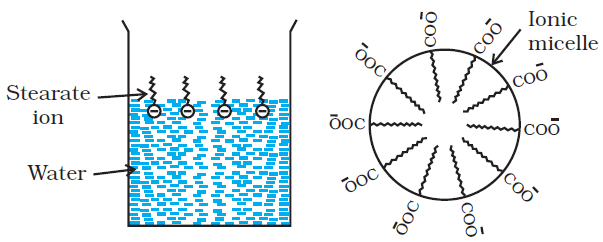
Cleansing action of soaps :-
The cleansing action of soap is due to the fact that soap molecules form micelle around the oil droplet in such a way that hydrophobic part of the stearate ions is in the oil droplet and is in the oil droplet and hydrophilic part projects out of the gresse droplet like the bristles.

Since the polar group can interact with water, the Oil droplet surrounded by stearate ions is now pulled in water and removed from the dirty surface.
Thus soap helps in emulsification and washing away of oils and fats. The negatively charged sheath around the globules prevents them from coming together and forming aggregates.
Preparation of Colloids :-
( a ) Chemical methods :-
Colloidal Solutions can be prepared by chemical reactions leading to formation of molecules by double decomposition, oxidation, reduction or hydrolysis. These molecules then aggregate leading to formation of sols.

( b ) Electrical disintegration or Bredig’s Arc method :-
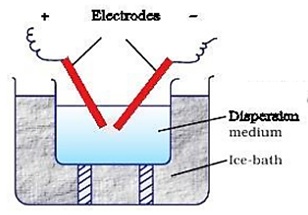
This process involves dispersion as well as condensation. Colloidal sols of metals such as gold, silver, Platinum etc. can be prepared by this method. In this method, electric arc is struck between electrodes of the metal immerged in the dispersion medium. The intense heat produced vaporises the metal, which the condenses to form particles of Colloidal size.
( c ) Peptization :-
Peptization may be defined as the process of converting a precipitate into Colloidal sol by shaking it with dispersion medium in the presence of a small amount of electrolyte.
- The electrolyte used for this purpose is called peptizing agent. This method is applied generally, to convert a freshly prepared precipitate into a colloidal Sol.
- During peptization, the precipitate adsorbs one of the ions of the electrolyte on its surface. This causes the development of positive or negative charge on precipitate, which ultimately break up into smaller particles of the size of a Colloid.
Purification of Colloidal Solutions :-
The process used for reducing the amount of impurities to a requisite minimum is known as purification of colloidal solution.
The purification of colloidal solution is carried out by the following methods :
( 1 ) Dialysis :-
It is a process of removing a dissolved substance from a colloidal solution by means of diffusion through a suitable membrane.
Since particles ( ions or small molecules ) in a true solution can pass through animal membrane ( bladder ) or parchment paper or cellophane sheet but not the colloidal particles, the membrane can be used for dialysis.
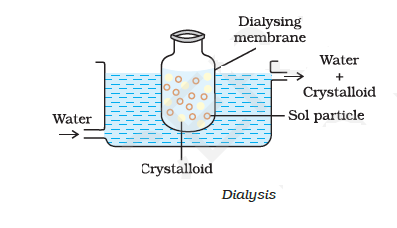
The apparatus used for this purpose is called dialyzer.
- A bag of suitable membrane containing the colloidal solution is suspended in a vessel through which fresh water is continuously flowing.
The molecules and ions diffuse through membrane into the outer water and pure colloidal solution is left behind.
( 2 ) Electro-dialysis :-
The process of dialysis is quite slow. It can be made faster by applying and electric field if the dissolved substance in the impure colloidal solution is only an electrolyte. The process is then named electrodialysis.
- The colloidal solution is placed in a bag of suitable membrane while pure water is taken out side.

- Electrodes are fitted in the compartment as shown in the above figure. The ions present in the colloidal solution migrate out of the oppositely charged electrodes.
( 3 ) Ultrafiltration :-
Ultrafiltration is the process of separating the colloidal particles from the solvent and soluble solutes present in the colloidal solution by a specially prepared filters, which are permeable to all substances except the colloidal particles.
- Colloidal particles can pass through ordinary filter paper because the pores are too large. However, the process of filter paper can be reduced in size by impregnating with colloidian solution to stop the flow of colloidal particles. the usual Colloidian is a 4% solution of nitrocellulose in a mixture of alcohol and ether.
- An ultrafilter paper may be prepared by soaking the filter paper in a colloidian solution, hardening by formaldehyde and then finally driying it.
Thus, by using Ultra filter paper, the colloidal particles are separated from rest of the materials. Ultrafiltration is a slow process.
Properties of Colloidal Solutions :-
Following properties exhibited by the Colloidal solutions –
( 1 ) Colligative properties :-
Colloidal particles being bigger aggregates, the number of particles in a colloidal solution is comparatively small as compared to a true solution. Hence, the values of colligative properties ( osmotic pressure, lowering in Vapour pressure, depression in freezing point and elevation in boiling point ) are of small order as compare to values shown by true solutions at same concentration.
( 2 ) Tyndall effect :-
If a homogeneous solution placed in dark is observed in the direction of light, it appears clear and, if it is observed from a direction at right angles of the direction of light beam, it appears perfectly dark.
- Colloidal solutions viewed in the same way may also appear reasonably clear or translucent by the transmitted light but they show a mild to strong opalescence, when viewed at right angles to the passage of light, i. e. the path of the beam is illuminated by a bluish light. This effect was first observed by Faraday and later studied in detail by Tyndall and is termed as Tyndall effect.

- The bright cone of light is called Tyndall cone. The Tyndall effect is due to the fact that the Colloidal particles scatter light in all directions in space. This scattering of light illuminates the path of beam in the Colloidal dispersion.
- Tyndall effect can be observed during the projection of picture in the cinema hall due to scattering of light by dust and smoke particles present there.
( 3 ) Colour :-
The colour of colloidal solution depends on the wavelength of light scattered by the dispersed particles. The wavelength of light further depends on the size and nature of the particles.
- The colour of colloidal solution also changes with the manner in which the observer receives the light.
For example, a mixture of milk and water appears blue when viewed by the reflected light and red when viewed by the transmitted light.
( 4 ) Brownian movement :-
When Colloidial solutions are viewed under a powerful ultramicroscope, the colloidal particles appear to be in a state of continuous zig-zag motion all over the field of view. This motion was first observed by the British botanist, Robert Brown and is known as Brownian movement.
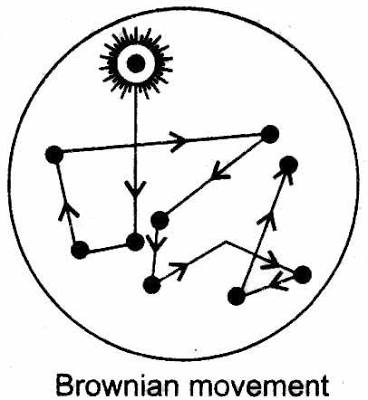
This motion is independent of the nature of colloid but depends on the size of the particles and viscosity of the solution.
Smaller the size and lesser the viscosity, faster is the motion.
( 5 ) Charge on Colloidal particles :-
Colloids particle always carry an electric charge. The nature of this charge is the same on all the particles in a given colloids solution and may be either positive or negative.
- The combination of two layers of opposite charges around the colloids particle is called Helmholtz electricalvehicle double layer.
- According to modern views, the first layer of ions is firmly held and is termed fixed layer while the second layer is mobile which is termed diffused layer. Since separation of charge is a seat of potential, the charges of opposite signs on the fixed and diffused parts of the double layer results in a difference in potential between these layers.
- This potential difference between fixed layer and the diffused layer of opposite charges is called the electrokinetic potential or Zeta potential
( 6 ) Electrophoresis :-
The existence of charge on colloidal particles is confirmed by electrophoresis experiment. When electric potential is applied across two Platinum electrodes dipping in a colloids solution, the colloidal particles move towards one or the other electrode. The movement of Colloidal particles under an applied electric potential is called electrophoresis.
- Positively charged particles move towards the cathode while negatively charged particles move towards the anode.
- When electrophoresis i. e. movement of particles is prevented by suitable means, it is observed that the dispersion medium begins to move in an electric field. This phenomenon is termed electro-osmosis.
( 7 ) Coagulation or Precipitation :-
The stability of the lyophobic sols is due to the presence of charge on colloidal particles. If the charge is removed, the particles will come nearer to each other to form aggregates or coagulation and settle down under the force of gravity.
Emulsions :-
If a mixture of two immiscible or partially miscible liquids is shaken, a dispersion of one liquid in the other is obtained which is called emulsion. Generally, one of the two liquids is water.
There are two types of emulsion –
- Oil dispersed in water and
- Water dispersed in oil. In the first system water acts as dispersion medium. Examples of this type of emulsion are milk and vanishing cream. In milk, liquid fat is dispersed in water. In the second system, Oil acts as dispersion medium. Common examples of this type of butter and cream. Emulsion of oil in water are unstable and sometimes they separated into two layers on standing.
Emulsions can be diluted with any amount of dispersion medium. They can be broken into constituent liquids by heating, freezing, centrifuging etc.
Applications of Colloids :-
Colloids are widely used in the industry.
Following are some examples –
- Electrical precipitation of smoke.
- Purification of drinking water.
- Medicines.
- Tanning.
- Cleansing action of soaps and detergents.
- Photographic plates and films.
- Rubber Industry.
- Industrial Products.








