Adsorption :-
Adsorption is defined as the deposition of molecular species onto the surface .
The molecular species that gets absorbed on the surface is known as absorbate and the surface on which adsorption occurs is known as adsorbent .

Common examples of adsorbents are clay , silica , gel , metal etc .
Adsorption in action :-
1 . If a gas like O₂ , H₂ Cl₂ , NH₃ or SO₂ is taken in a closed vessel containing powdered charcoal , it is observed that the pressure of the gas in the enclosed vessel decreases i . e . gas are adsorbed at the surface .
2 . In a solution of an organic dye , say methylene blue , when animal Charcoal is added and the solution is well shaken , it is observed that the filtrate turns colourless . The molecules of the dye , thus accumulate on the surface of charcoal .
3 . Aqueous solution of raw sugar , when passed over beds of animal charcoal , becomes colourless as the colouring substances are adsorbed by charcoal .
4 . The air becomes dry in the presence of Silica gel because the water molecules get adsorbed on the surface of the gel .
It is clear from above examples that solid surfaces can hold the gas or liquid molecules by virtue of adsorption .
Desorption :-
The process of removing an adsorbed substance from a surface on which it is adsorbed is called desorption .
Absorption :-
The process of absorption means that a substance captures and transforms energy . The absorbent distributes the material it captures throughout whole .

Example – water vapours are absorbed by anhydrous Calcium chloride .
- Absorption is a physical , chemical phenomenon or a process in which atoms, molecules or ions enter some bulk phase – liquid or solid .

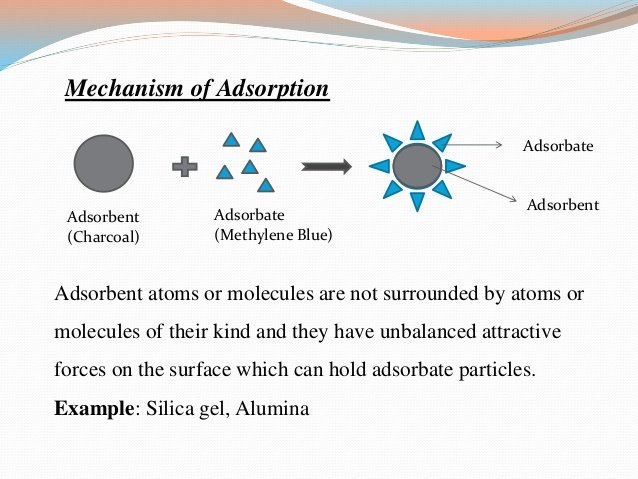
Mechanism of Adsorption :-
They arises due to the fact that the surface particles of the adsorbent are not in the same environment as the particles inside the bulk .
- Inside the adsorbent all the forces acting between the particles are mutually balanced but on the surface the particles are not surrounded by atoms or molecules of their kind on all sides and hence possess unbalanced or residual attractive forces .
- During adsorption , there is always a decrease in residual forces of the surface , i . e .there is decrease in surface energy which appears as heat . Therefore adsorption is invariably an exothermic process . ∆H of adsorption is always negative , Entropy ∆S and Gibbs energy ∆G can be negative .
On the basis of equation ∆G = ∆H – T ∆S
Types of Adsoption :-
( 1 ) Physical Adsorption or Physisorption
( 2 ) Chemical Adsorption
Physical Adsorption :-
If accumulation of gas on the surface of a solid occurs on account of weak Van der waals forces , It is termed as physical adsorption .
- A physical adsorption at low temperature may pass into chemical adsorption as the temperature is increased .
Example – dihydrogen is first adsorbed nickel by Van der waals forces . Molecules of hydrogen then dissociate to form hydrogen atoms which are held on the surface .
Characteristics of Physisorption :-
Lack of specificity :- A given surface of an adsorbent does not show any preference for a particular gas as the Van der waals forces are universal .
- Nature of adsorbate :- The amount of gas adsorbed by a solid depends on the nature of gas . In general easily liquefiable gases are readily adsorbed as Van der waals forces are stronger near the critical temperature .
Reversible nature :- Physisorption of a gas by a solid is generally reversible .
Since the adsorption process is exothermic , the physisorption occurs readly at low temperature and decreases with increasing temperature ( Me Chatelier’s principle )
- Surface area of adsorbent :- The extent of adsorption increases with the increase of surface area of the adsorbent .
Thus , finally divided metals and porous substances having large surface areas are good at adsorbents .
- Enthalpy of adsorption :- Physisorption is an exothermic process but it enthalpy of adsorption is quite low ( 20 to 40 kilo joule per mole ) . This is because the attraction between gas and solid molecules is only due to weak Van der waals forces .
Chemisorption or Chemical Adsorption :-
When the gas molecules or atoms are held to the solid surface by chemical bonds , the adsorption is termed chemisorption .
- The chemical bonds may be covalent or ionic bond .
- Chemisorption involves a high energy of activation and is therefore , often referred to gas activated adsorption .
Example – molecules of hydrogen dissociate to form hydrogen atoms which are held on the surface of nickel by chemisorption .
Characteristic of chemisorption :-
- High specificity :- It is highly specific and it will only occur if there is some possibility of chemical bonding between adsorbent and adsorbate .
Example – Oxygen is adsorbed on metals by virtue of Oxide formation .
- Irreversibility :- As chemisorption involves compound formation , it is usually irreversible in nature . It is also an exothermic process but the process is very slow at low temperature .
- Surface area :- Like physisorption , it also increases with increase of surface area of the adsorbent .
- Enthalpy of adsorption :- Enthalpy of chemisorption is high ( 82 to 240 kilo joule per mole ) as it involves chemical bond formation .
Adsorption Isotherms :-
The variation in the amount of gas adsorbed by the adsorbent with pressure at constant temperature can be expressed by means of a curve termed as adsorption Isotherm .
Freundlich Adsorption Isotherm :-
In 1909 , he gave an empirical relationship between the quantity of gas adsorbed by unit mass of solid adsorbent and pressure at a particular temperature .
The relationship can be expressed by the following equation –
x / m = K P¹/ⁿ ( n > 1 )
Where x is the mass of the gas adsorbed on mass m of the adsorbent at pressure P , K and n are constants which depend on nature of the adsorbent and the gas at a particular temperature .
The factor 1/n can have values between 0 and 1 .

When 1/ n = 0 then x / m = constant
1 / n = 1 then x / m = K P

Taking logarithm of equation –

Adsorption from solution phase :-
When a solution of acetic acid in water is shaken with charcoal , a part of the acid is adsorbed by the charcoal and the concentration of the acid decreases in the solution .
The following observations have been made in the case of adsorption from solution phase .
1 . The extent of adsorption decreases with an increase in temperature .
2 . The extent of adsorption increases with an increase of surface area of the adsorbent .
3 . The extent of adsorption depends on the concentration of the solute in the solution .
4 . The extent of adsorption depends on the nature of the adsorbent and the adsorbate .
Catalysis :-
Substances , which alter the rate of a chemical reaction and themselves remain chemically and quantitatively unchanged after the reaction are known as catalysts , and the phenomenon is known as Catalysis .
Catalysis can be divided into two groups :-
1 . Homogeneous Catalysis .
2 . Heterogeneous Catalysis .
Homogeneous Catalysis :-
When the reactants and catalyst are in the same phase i . e . liquid or gas , the process is said to be homogeneous catalysis .
Examples –

Heterogeneous catalysis :-
The catalytic process in which the reactants and the catalyst are in different phase is known as heterogeneous Catalysis .
Examples –

Adsorption theory of heterogeneous catalysis :-
This theory explains the mechanism of heterogeneous Catalysis . The catalytic action can be explained in terms of the intermediate compound formation , the modern theory is the combination of intermediate compound formation theory and the old theory . The old theory states that the reactants in gaseous state or in solutions are adsorbed on the surface of the solid Catalyst .
- The mechanism involves five steps –
1 . Diffusion of reactants to the surface of the catalyst .
2 . Adsorption of reactant molecules on the surface of the catalyst .
3 . Occurrence of chemical reaction on the catalyst’s surface through formation of an intermediate .
4 . Desorption of reaction products from the catalyst surface and thereby , making the surface available again for more reaction to occur .
5 . Diffusion of reaction products away from the Catalyst’s surface .

Important features of solid catalysts :-
( a ) Activity :- The activity of a catalyst depends upon the strength of chemisorption to a large extent . The reactants must get adsorbed reasonably strongly on the catalyst to become active . However , they must not get adsirbed so strongly that they are immobilised and other reactants are left with no space on the catalyst’s surface for adsorption .
- It has been found that for hydrogenation reaction , the catalytic activity increases from group 5 to group 11 metals with maximum activity being show by groups 7 to 9 elements of the periodic table .

( b ) Selectivity :- The selectivity of a catalyst is its ability to direct a reaction to yield a particular product selectively , selectivity of different catalysts for same reactants is different .
Examples –

Shape selective Catalysis by Zeolites :-
The catalytic reaction that depends upon the pore structure of the catalyst , the size of reactant and product molecules is called shape selective catalysis .
- Zeolites are group shape – selective catalysts because of their honeycomb like structures . They are microporous aluminosilicates with three-dimensional network of silicates in which some Silicon atoms are replaced by aluminium atoms give Al – O – Si framework .
- Zeolites are being very widely used as catalysts in petrochemical industries for cracking of hydrocarbons and isomerisation .
Enzyme Catalysis :-
Enzymes are complex nitrogenous organic compounds which are produced by living plants and animals . They are proteins . Many reactions that occur in the bodies of animals and plants to maintain the life process are catalysed by enzymes .
The enzymes are thus , termed as biochemical catalysts and the phenomenon is known and biochemical catalysis .
Examples of enzyme catalysed reactions :-


Characteristics of Enzyme catalysis :-
1 . One molecule of an enzyme main transform 1 million molecules of the reactant per minute .
2 . Each enzyme is specific for a given reaction i . e . one catalyst cannot catalyse more than one reaction .
3 . The rate of enzyme reaction becomes maximum at a definite temperature called the optimum temperature ( 298 to 320 K ) .
4 . The rate of an enzyme catalysed reaction is maximum at a particular pH called optimum pH ( value 5 to 7 ) .
5 . The enzymatic activity is increased in presence of certain substances , known as co- enzymes .
6 . Like ordinary catalyst , enzymes are also inhibited or poisoned by the presence of certain substances .
Mechanism of Enzyme catalysis :-
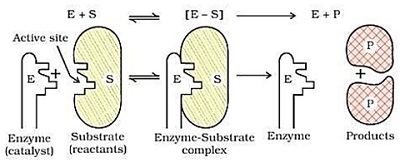
There are a number of cavities present on the surface of colloidal particles of enzymes , these cavities are of characteristic shape and posses active groups such as – NH₂ , – COOH , – SH , – OH etc .
The molecules of the substance ( reactant ) which have complementary shape , fit into these cavity just like a key fit into a lock .
On account of the presence of active groups , an activated complex is formed which then decomposed to yield the product .
Thus , the enzyme catalysed reactions may be considered to proceed in two steps –
step 1 –
Binding of enzyme to substance to form an activated complex .
E + S → E S
step 2 –
Decomposition of the activated complex to form product .
E S → E + S
Catalysts in industry :-