Magnetic properties of Crystals :-
Each electron in an atom behaves like a magnet. It’s magnetic moment originates from two types of motions are orbital motion around the nucleus and spin around its own axis. Magnitude of magnetic moment is very small and is measured in the unit called Bohr magneton μB. It is equal to 9·27 x 10⁻²⁴ A m²

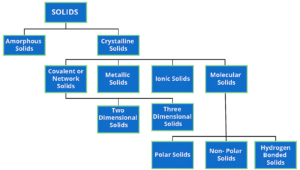
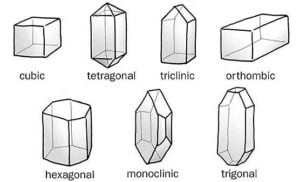
On the basis of their magnetic properties Substances can be classified into five categories. –
1. Paramagnetic Substance :-
Pramagnetic substaces are weakly attracted by a magnetic field. They are magnetised in magnetic field in the same direction.
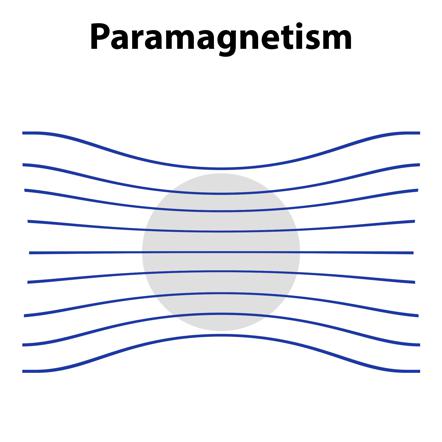
- They lose their magnetism in the absence of magnetic field.
- Paramagnetism is due to presence of one or more unpaired electrons which are attracted by the magnetic field.
Examples. – O² , Cu²⁺ , Fe³⁺ , Cr³⁺ , Ni
2. Diamagnetic Substance :-
They are weakly repelled by a magnetic field. They are weakly magnetised in a magnetic field in opposite direction.
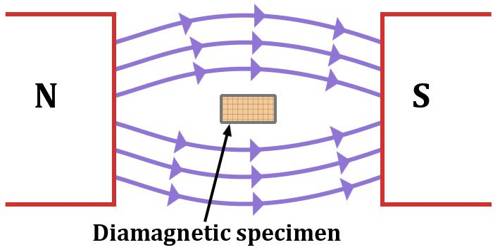
- Diamagnetism is shown by those substaces in which all the electrons are paired and there are no unpaired electrons.
- Pairing of ekectrons cancels their magnetic moments and they lose their magnetic character or magnetism.
Example.- H₂O , NaCl , C₆H₆ , Ne
Also read :- Defects in Crystals
3. Ferromagnetic Substance :-
Ferromagnetic substances are attracted very strongly by a magnetic field. Besides strong attractions , thes substaces can be permanently magnetised.
- They are grouped togather into small regions called domais , each domains acts as a tiny magnet.
- When the substance is placed in a magnetic field all the domains get oriented in the direction of the magnetic field.
- They are a permanent magnet.
Example :- Fe , Co , Ni , CrO₂
4. Antiferromagnetic Substances :-
Antiferromagnetic have domain structure similar to ferromagnetic but their domains are oppositely oriented and cancel out each other’s magnetic moment.
Examples. – MnO
5. Ferrimagnetic Substance :-
It is observed when the magnetic moments of the domains in the substance are aligned in parallel and anti parallel conditions in unequal numbers. They are weakly atracted by magnetic field as compared to Ferromagnetic.
Examples. – MgFe₂O₄ , Fe₃O₄ , ZnFe₂O₄