Structure of Atomic Nucleus :-
The atomic nucleus is the small, dense region consisting of protons and neutrons at the centre of an atom, discovered in 1911 by Ernest Rutherford based on the Geiger Marsden gold foil experiment.
- Protons and Neutrons are bound together to form a nucleus by the nuclear force.
- Radius of nucleus is about 1.2 × 10⁻¹⁵ m.
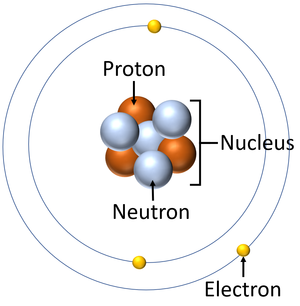
Proton :-
A Proton is a subatomic particle, symbol ‘p‘ or p⁺ with a positive charge of +1e (elementary charge) and mass slightly less than that of a Neutron.
- Protons and Neutrons, each with masses of approximately one atomic mass unit are collectively referred to as nucleons.
- One or more protons are present in the nucleus of every atom. They are a necessary part of the nucleus. The numbers of proton ‘p’ = atomic number (Z)
Properties of Proton :-
- Discovered by Goldstein in 1886
- Symbol —– p, p⁺, N⁺, ₁¹H⁺
- Statistics —– Fermionic.
- Interactions —– Gravity, electromagnetic.
- Mass —— 1·672621 x 10⁻²⁷ kg, 1·007276 u
- Mean lifetime —— > 2·1 x10²⁹ years
- Electric charge —— +1e, 1·602 x 10⁻¹⁹ C
- Electric dipole moment —— < 5·4 x 10⁻²⁴ e⁻ᶜᵐ
- Electric polarizability ——– 1·2 x 10⁻³ f m³
- Magnetic moment ——- 1·41 x 10⁻²⁶ J T⁻¹
- Spin ————- 1/2
- Isospin ———- 1/2
Neutron :-
The neutron is a subatomic particle, Symbol ‘n’ with no net electric charge and a mass slightly greater than that of a proton.
Properties of Neutron :-
- Discovered by James Chadwick in 1932.
- Symbol ——- n, n⁰, N⁰
- Statistics ——- Fermionic.
- Interactions ——– Gravity, electromagnetic.
- Mass ——– 1·674927 x 10⁻²⁷ kg, 1·008664 u
- Mean lifetime ——– 881·5 second
- Electric charge ——- zero
- Electric dipole moment ——– < 2·9 x 10⁻26 e cm
- Spin ———- 1/2
- Isospin ———- -1/2
A free neutron, unlike a free Proton is unstable. It decays into a Proton, an electron and antineutrino (another elementary particle). It is however stable inside the nucleus.
The composition of a Nucleus :-
Z → atomic number = number of protons.
N → neutron number
A → mass number = Z + N
= Total number of protons and neutrons
i.e. A = Z+N Or N = A – Z
Example – The nucleus of gold is denoted by
¹⁹⁷₇₉Au
Here it contains 197 nucleons of which 79 are protons and the rest are neutrons.
- All the electrons of an atom are outside the nucleus. we know that the number of these electrons outside the nucleus of the atom is Z, the atomic number.
The total charge of the atomic electrons is thus – Ze and since the atom is neutral. The charge of the neutron in + Ze .
The number of protons in the nucleus of the atom is therefore, exactly Z, the atomic number.
Size of Atomic Nucleus :-
BY performing scattering experiments in which fast electrons, instead of α – particles are projectiles that Bombard targets made up of various elements, the size of nuclei of various elements have been accurately measured.
It has been found that a nucleus of mass number ‘A’ has radius
R = R₀ A¹/³
Where R₀ = 1·2 x 10⁻¹⁵ m
This means the volume of the nucleus, which is proportional to R³ is proportional to ‘A’
i.e. V α A or 4/3 πR³ α A
Or R³ α A or R α A¹/³
Or R = R₀ A¹/³