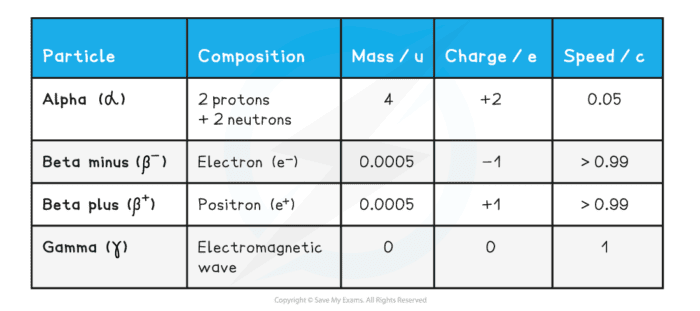
Properties of Alpha Beta and Gamma Rays :-
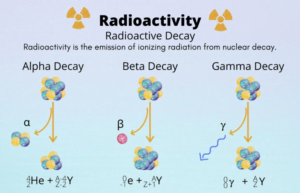
During radioactive decay, particles like alpha, beta and gamma rays are emitted by the unstable atoms like uranium, thorium, polonium, radium, actenium etc to gain stability.
- Properties of alpha beta and gamma rays are given below –
Properties of Alpha Rays :-
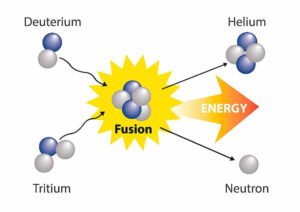
- Alpha Rays are the positively charged particles, A highly energetic helium nucleus which contains two protons and two neutrons is called the Alpha particle.
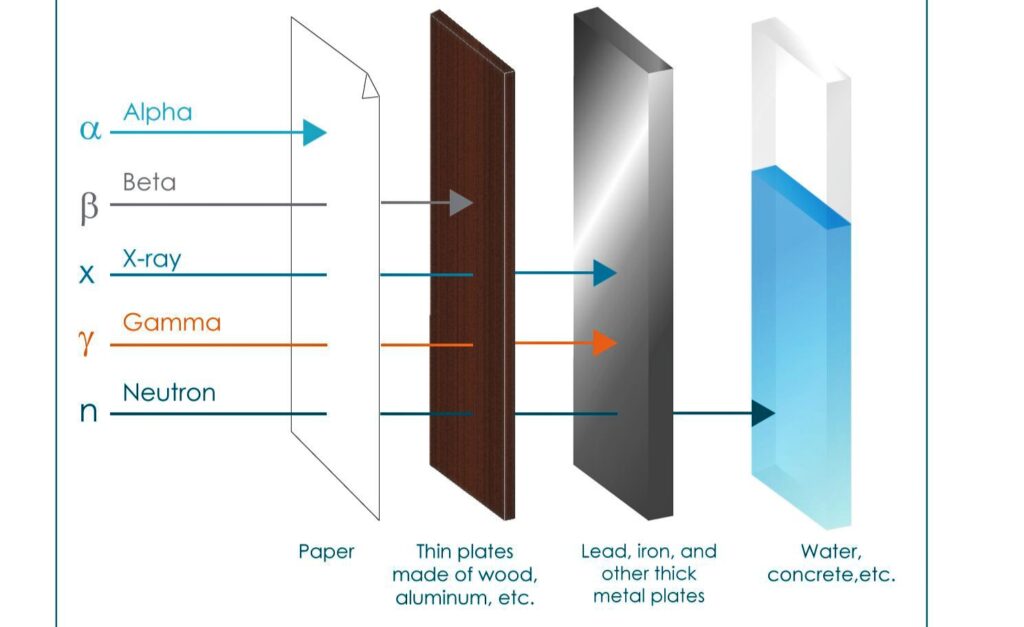
- Alpha rays have the least penetrating power but the greatest ionization power. It is about 100 times of Beta and about 10000 Times of gamma.
- They are affected by both electric and magnetic field.
- Their effects on photographicgraphic plate.
- Its speed is less than the speed of light.
- They are not dangerous to contact with the body.
Properties of Beta Rays :-
- They have negatively charged particles and have a negligible mass . It is an electron that is ejected from the nucleus at high speed.
- Beta particles have greater penetrating power than the Alpha particles.
- They have less ionization power than the Alpha particles. they are affected by both electric and magnetic field.
- They are more effective than Alpha particle on photographic plate.
- Its speed is equal to the speed of light.
- They are dangerous and so their contact with the body must be avoided.
Properties of Gamma Rays :-
- They are electromagnetic wave like X -ray, its wavelength is 1/100 of X -rays wavelength. They are known as Gamma Photon.
- Its energy is very high.
- They are not affected by both electric and magnetic field.
- They propagate from the speed of light.
- Its penetrating power is more than Alpha particles and beta particles.
- When Gamma ray falls on metal surface, then emits photoelectron.
- They effects on photographic plate more than beta particles.

alpha all daitel