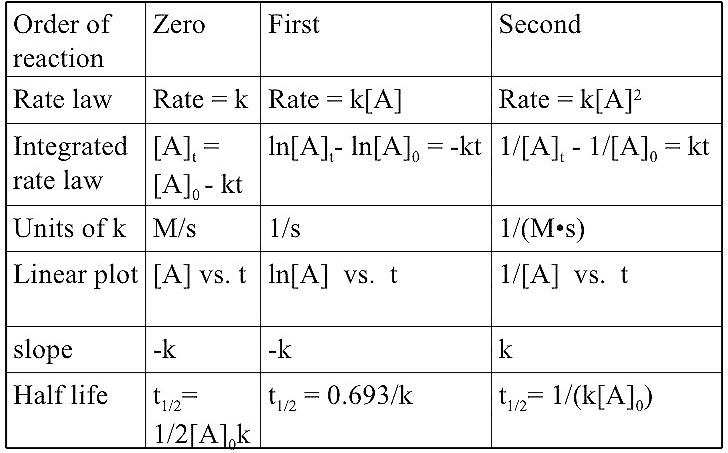
Order of Reaction :-
The sum of powers of concentration of the reactants in the rate expression is called the order of that chemical reaction .
Example – Rate of reaction = K [ A ]ˣ [ B ]ʸorder
Here x and y represent the order with respect to the reactants A and B respectively . Sum of these components i . e . x + y gives the overall order of a reaction .
Order of a reaction can be 0 , 1 , 2 , 3 and even a fraction . A zero order reaction means that the rate of reaction is independent of the concentration of reactants .
Question – Calculate the overall order of reaction which has the rate expression
( a ) Rate = K [ A ]⁰·⁵ [ B ]¹·⁵
( b ) Rate = K [ A ]¹·⁵ [ B ]⁻¹
Solution –
( a ) Rate = K [ A ]ˣ [ B ]ʸ
Order = x + y = 0 · 5 + 1·5 = 2
i . e . second order of reaction .
( b ) Order = 1·5 + ( – 1 ) = 0·5 or 1 / 2
i . e . Half order of reaction .
Units of Rate Constant :-
For a general reaction
aA + bB → cC + dD
Rate = K [ A ]ˣ [ B ]ʸ
Where x+ y = n = Order if reaction .
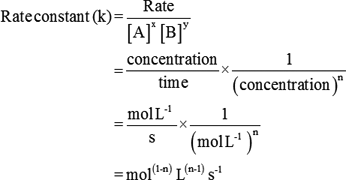
Where A = B
Taking SI units of concentration , mol / L⁻¹ and time s , the units of K for different reaction order are given –
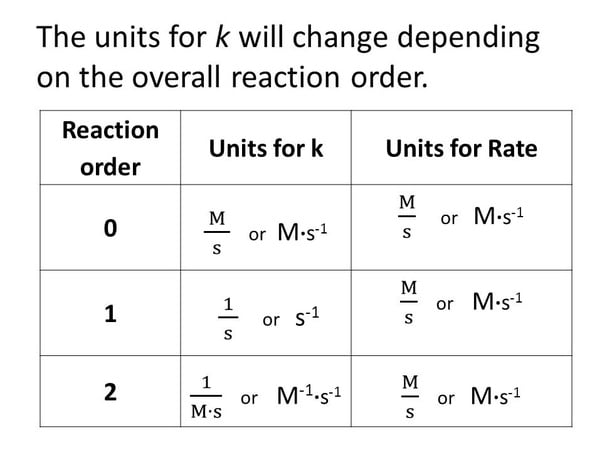
Here M = mol L⁻¹
Question – Identify the reaction order from each of the following rate constants
1 . K = 2·3 x 10⁻⁵ L mol⁻¹ s⁻¹
2 . K = 3 x 10⁻⁴ s⁻¹
Solution –
1 . The unit of second order rate constant is L mol⁻¹ s⁻¹
Therefore K = 2·3 x 10⁻⁵ L mol⁻¹ s⁻¹ represents a second order reaction .
2 . The unit of first order rate constant is s⁻¹ , therefore K = 3 X 10⁻⁴ s⁻¹ represents a first order reaction .
Zero Order of Reaction :-
The rate of the reaction is proportional to zero power of the concentration of reactants is called zero order reaction .
Considered the reaction ,
R → P
Rate = d[ R ] / dt = K [ R ]⁰
As any quantity raise to power 0 is unity .
Rate = – d[ R ] / dt = K x 1
d [ R ] = – K dt
Integrating both sides
[ R ] = – K t + I —————– ( 1 )
Where I is the constant of integration .
At t = 0 , the concentration of the reactant
R = [ R ]₀
Where [ R ]₀ is initial concentration of the reactant .
Substituting in equation ( 1 )
[ R ]₀ = – K x 0 +I
[ R ]₀ = I
Substituting the value I in equation ( 1 )
[ R ] = – Kt + [ R ]₀
Concentration Vs time plot for zero order reaction :-
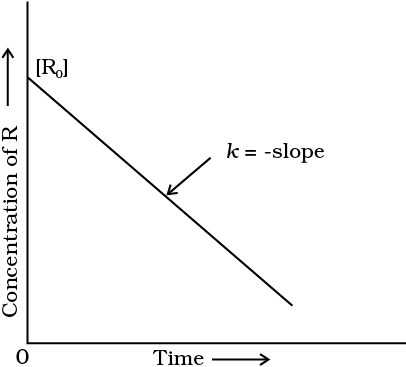
If we plot [ R ] against t , we get a straight line with slope = – K and intercept equal to [ R ]₀
Rate constant for zero order of reaction :-
Simplifying equation [ R ] = – K t + [ R ]₀
We get the rate constant , K as
K = [ R ]₀ – [ R ] / t
Some enzyme catalysed reactions and reactions which occur on metal surfaces are a few examples of zero order reactions .
1 . The decomposition of gaseous ammonia on a hot Platinum surface is zero order reaction at high pressure –
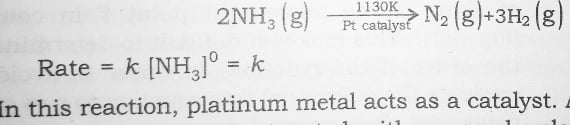
2 . The thermal decomposition of HI on gold surface is another example of zero order reaction .
First Order of Reaction :-
The rate of reaction is proportional to the first power of the concentration of the reactant R is called first order of reaction .
Example –
R → P
a 0 t = 0
a – x x after time t
or d[ R ] / [ R ] = – K dt
Integrating this equation , we get
In [ R ] = – K t + I —————– ( 1 )
Where I is integration constant .
When t = 0 , R = [ R ]₀
Where [ R ]₀ is the initial concentration of the reactant .
Then
In [ R ]₀ = – K x 0 + I
In [ R ]₀ = I
Substituting the value o I in equatiin ( 1 )
In [ R ] = – K t + In [ R ]₀
Or In R / [ R ]₀ = – K t
Or
K = (1 / t ) In [ R ]₀ / [ R ]
The first order rate equation can be written in the form of
K = ( 1 / t ) In a / a – x
Or K = ( 2·303 / t ) log a / a – x
Or K = ( 2·303 / t ) log [ R ]₀ / [ R ]
- If [ R ]₁ and [ R ]₂ are the concentrations of the reactant at time T₁ and t₂ respectively then subtracting equation
In [ R ]₂ = – K t₂ + In [ R ]₀
From In [ R ]₁ = – K t₁ + In [ R ]₀
We get
In [ R ]₁ – In [ R ]₂ = – K t₁ – ( – K t₂ )
In [ R ]₁ / [ R ]₂ = K ( t₂ – t₁ )
K = ( 1 / t₂ – t₁ ) In [ R ]₁ / [ R ]₂
Or K = ( 2·303 / t₂ – t₁ ) log ( a – x₁ / a – x₂ )
- Equation ln [ R ] = – K t + In [ R ]₀ can also be written as
In [ R ] / [ R ]₀ = – K t
Taking antilog of both sides
[ R ] =[ R ]₀ e⁻ᵏᵗ
Concentration [ R ] and log [ R ]₀ / [ R ] vs time ( t ) plot for a first order reaction :-
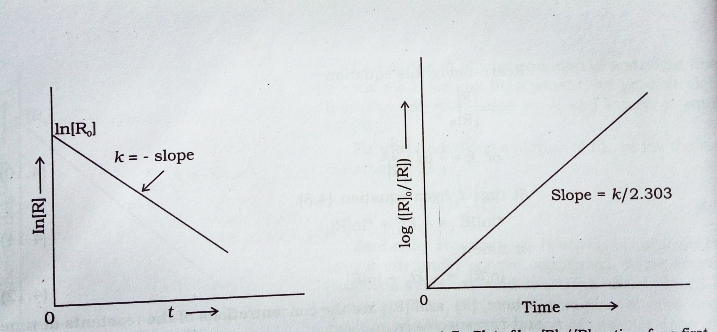
If we plot In [ R ] against t we get a straight line with slope = – K and intercept equal to In [ R ]₀
Decomposition of N₂O₅ and N₂O are some more examples of first order reactions .
Question – The initial concentration of N₂O₅ in the following first order reaction N₂O₅ ( g ) → 2NO₂ ( g ) + 1/2O₂ ( g ) was 1.24 x 10⁻² mole L⁻¹ at 318 K .
The concentration of N₂O₅ after 60 minutes was 0.20 x 10₋₂mole per litre , calculate the rate constant of the reaction at 318 k
Solution –
For a first order reaction
K = ( 2·303 / t₂ – t₁ ) log [ R ]₁ / [ R ]₂
= ( 2·303 / 60 min – 0 min ) log 1·24 x 10⁻² mol L⁻¹ / 0·20 x 10⁻² mol L⁻¹
= ( 2·303 / 60 ) log 6·2 min⁻¹
K = 0·0304 min⁻¹
Half life of a reaction :-
The half life of reaction is the time in which the concentration of reactant is reduced to one half of its initial concentration , it is represented as t₁/₂
- For the zero order reaction , rate constant is given by equation
K = [ R ]₀ – [ R ] / t
At t = t₁/₂ , [ R ] = 1/2 [ R ]₀
The rate constant at t₁/₂ becomes
K = [ R ]₀ – 1/2 [ R ]₀ / t₁/₂
t₁/₂ = [ R ]₀ / 2 K
t₁/₂ for a zero order reaction is directly proportional to the initial concentration of the reactants and inversely proportional to the rate constant .
- For the first order reaction ,
K = ( 2·303 / t ) log [ R ]₀ / [ R ]
At t₁/₂ , [ R ] = [ R ]₀ / 2
So , the above equation becomes
K = ( 2·303 / t₁/₂ ) log [ R ]₀ / R ]₀ / 2
Or t₁/₂ = ( 2·303 / K ) log 2
t₁/₂ = ( 2·303 / K ) x 0·301
t₁/₂ = 0·693 / K
Thus , half life period of first order reaction is independent of initial concentration of the reacting species . It is depend on rate constant .
Question :- A first order reaction is found to have a rate constant k = 5 · 5 x 10⁻¹⁴ s⁻¹ . Find the half life of the reaction .
Solution –
t₁/₂ = 0·693 / K = 0·693 / 5·5 x 10⁻¹⁴ s⁻¹
= 1·26 x 10¹⁴ s
Question :- Show that a first order reaction , time required for completion of 99.9% is 10 times of half life ( t ₁/₂ ) of the reaction .
Solution –
When reaction is completed 99.9%
[ R ]ₙ = [ R ]₀ – 0·999[ R ]₀
K = ( 2·303 / t ) log [ R ]₀ / [ R ]
= ( 2·303 / t ) log [ R ]₀ / [ R ]₀ – 0·999 [ R ]₀
= ( 2·303 / t ) log 10
t = 6·909 / K
For half life of the reaction
t₁/₂ = 0·693 / K
t / t₁/₂ = ( 6·909 / K ) x ( K / 0·693 ) = 10
Pseudo first Order of Reaction :-
A Pseudo first order of reaction can be defined as a second order of reaction that is made to behave like a first order of reaction .
This reaction occurs in one reacting material is present in great excess or in maintained at a constant concentration compared with the other substance .
Example –
A + B → C + D
This reaction is dependent upon the concentrations of both A and B but one of the components is present in large excess and the concentration of B is very high as compared to that of A .
The reaction is considered to be Pseudo first order of reaction with respect to A .
If component A is in large excess and the concentration of A is very high as compared to that of B , the reaction is considered to be Pseudo first order with respect to B .
Example 1 –
Hydrolysis of 0.01 mole of Ethyl acetate with 10 mole of water
CH₃COOC₂H₅ + H₂O → CH₃COOH +C₂H₅OH
t = 0 0·01 mol 10 mol 0 mol 0 mol
t 0 mol 9·9 mol 0·01 mol 0·01 mol The concentration of water does not get altered during the course of the reaction .
so , in the rate equation
Rate = K’ [ CH₃COOC₂H₅ ] [ H₂O ]
The term [ H₂O ] can be taken as constant .
The equation , thus becomes
Rate = K [ CH₃COOC₂H₅ ]
where K = K’ [ H₂O ] and the reaction behaves as first order of reaction , such reactions are called Pseudo first order of reactions .
Example 2 –
Inversion of Cane sugar is another Pseudo first order reaction .
C₁₂H₂₂O₁₁ + H₂O → C₆H₁₂O₆ + C₆H₁₂O₆
Cane sugar Glucose Fructose
Rate = K [ C₁₂H₂₂O₁₁ ]
Concentration Vs time plot for a Pseudo order of reaction :-
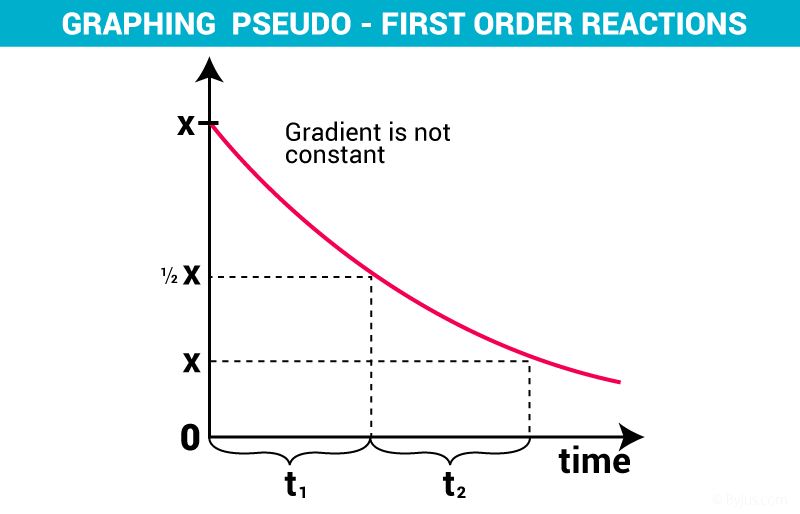
Elementary reaction :-
The reactions taking place in one step are called elementary reactions .
Complex reaction :-
When the reaction taking place more than one step or a sequence of elementary reactions ( called mechanism ) gives us the products , the reactions are called Complex reactions .
- These may be ( 1 ) Consecutive reactions
Example – Oxidation of Ethane to CO₂ and H₂O passes through a series of intermediate steps in which alcohol , aldehyde and acid are formed .
( 2 ) Reverse reactions
( 3 ) Side reactions
Example – Nitration of phenol yields o – nitro phenol and p – nitrophenol