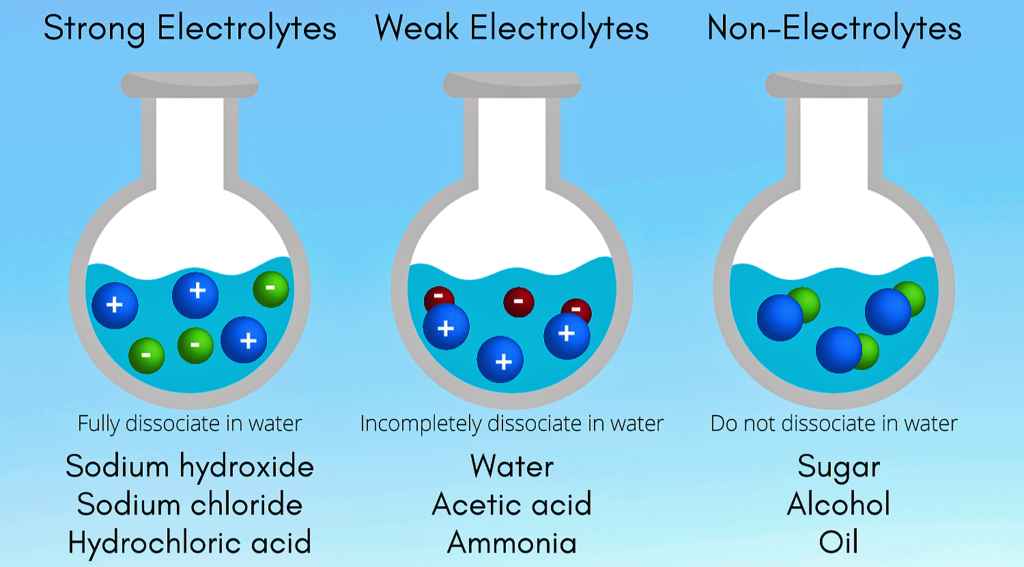
Electrolytes :-
Electrolytes are substances that produce an electrically conducting solution when dissolved in a polar solvent , such as water . The dissolved electrolytes separates into cations and anions , which disperse uniformly through the solvent .
Examples – NaCl , HCl , H₂SO₄ , HNO₃ , CaCl₂ , NaOH , CH₃COOH , KNO₃ etc .
Strong Electrolytes :-
Strong electrolytes are solutes or solutions I.e. electrolytes that completely dissociates in solution . The solution will contain only ions and no molecules of the electrolytes .
- Strong electrolytes are good conductors of electricity but only in aqueous solution .
Examples – NaCl , HCl , H₂SO₄ , HNO₃ , CaCl₂
Weak Electrolytes :-
Weak electrolytes are electrolytes that does not completely dissociate in aqueous solution . The solution will contain both ions and molecules of the electrolytes .
- Weak electrolytes are only partially ionize in water usually 1 % to 10%
Examples – CH₃COOH , NH₄OH , H₃PO₄ , H₂CO₃ etc .
Non-electrolytes :-
Non-electrolytes are substance that does not exist in an ionic form in aqueous solution . Non-electrolytes tend to be poor electrical conductors and don’t readily dissociate into ions when dissolved .
Example – Ethanol , Sugar , Chloroform .
Electrolysis :-
It is a technique that uses a direct electric current ( DC ) to drive an otherwise non -spontaneous chemical reaction .
- The site where electrolysis occurs is in an electrolytic cell , which is a type of electrochemical cell .
Electrolytic cell :-
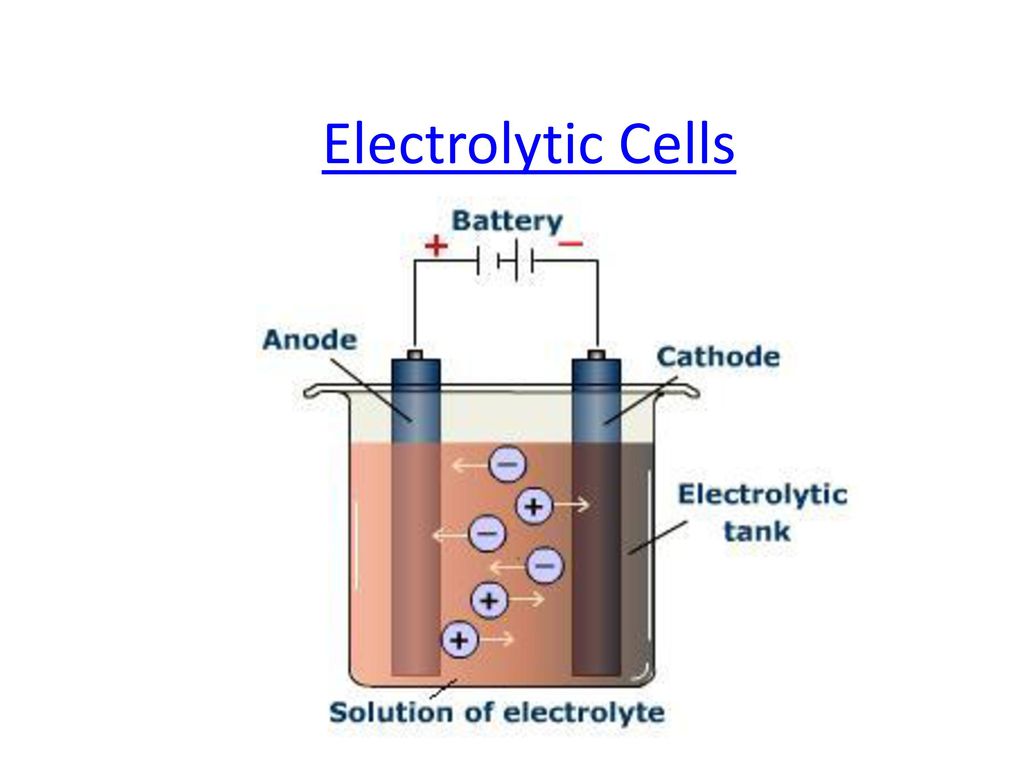
In an electrolytic cell external source of voltage is used to bring about a chemical reaction . The electrochemical process are of great importance in the laboratory and chemical industry .
- One of the simplest electrolytic cell consists of two copper strips dipping in an aqueous solution of copper sulphate .
- If a DC voltage is applied to the two electrodes , then Cu²⁺ ions discharge at the cathode ( negative charged ) and the following reaction takes place –
Cu²⁺ (aq ) + 2e⁻ → Cu ( s )
Copper metal is deposited on the cathode . At the anode , Copper is converted into Cu²⁺ by the reaction –
Cu ( s ) → Cu²⁺ ( s ) + 2e⁻
Thus Copper is dissolved ( oxidised ) at anode and deposited ( reduced ) at cathode .
- This is the basis for an industrial process in which impure copper is converted into copper of high purity .
- Sodium and magnesium metals are produced by the electrolysis of their fused chlorides and Aluminium is produced by electrolysis of Aluminium oxide in presence of cryolite .
Example – Electrolysis of liquified NaCl
NaCl → Na⁺ + Cl⁻
2Na⁺ + 2e → 2Na ( on cathode ) , Reduction
2 Cl⁻ → Cl₂ + 2e ( on anode ) , Oxidation
2Na⁺ + 2Cl⁻ → 2 Na + Cl₂
Faraday’s laws of Electrolysis :-
Michael Faraday was the first scientist who describe the quantitative aspects of electrolysis .
Faraday proposed two laws called Faraday’s law of electrolysis
First law :-
The mass of the substance deposited or liberated at any electrode is directly proportional to the quantity of electricity or charge passed .
In the mathematical form this law can be represented as follows
m α Q
m = ZQ
where m is the mass of substance in gram ( g ) , Q is charge measured in coulombs ( C ) and Z is the proportionality constant in g / C and is also known as electrochemical equivalent .
Electrochemical equivalent :-
It is the mass of substance produced at the electrode during electrolysis by one coulomb of charge I . e .
Z = m / Q
Since Q = It
Where I is current at time t
Hence m = ZQ
Or m = Z I t
If Equivalent weight of a substance is E , then
Z = E / 96500
Or. m = W = E I t / 96500
Second law :-
It states that – when the same quantity of electricity is passed through different electrolytes , the masses of different ions liberated at the electrodes are directly proportional to their chemical equivalents ( equivalent weights ) that means –
The electrochemical equivalent ( Z )of an element is directly proportional to its Equivalent weight ( E )
Or Z α E
1 Faraday ( F ) = charge of an electron × Avogadro’s number
Or 1F = 1.6021 x 10⁻¹⁹ C x 6.02 x 10²³ mol⁻¹
= 96487 or 96500
One mole of different ions liberated at the electrodes required amount of current electricity = n F
Where n is the valency bof ions .
Thus
1 mole of Ag⁺ = 1 x F = F
1 mole of Cu²⁺ = 2 x F = 2F
1 mole of Al³⁺ = 3 x F = 3F
1 mole of Mg²⁺ = 2 x F = 2F
For the electrode reaction
Ag⁺ + e → Ag
Cu²⁺ + 2e → Cu
Al³⁺ + 3e → Al
Mg²⁺ + 2e → Mg
Electrochemical cell :-
It is a device capable of either generating electrical energy from chemical reactions .
Examples – Galvanic cell and voltaic Cell and Daniel cell .
Galvanic cell or voltaic cell :-
A Galvanic cell is an Electrochemical cell that converts the chemical energy of a spontaneous Redox reaction into electrical energy . In this device the Gibbs energy of the spontaneous Redox reaction is converted into electrical work which may be used for running a motor or other electrical gadgets like heater , fan , geyser etc .
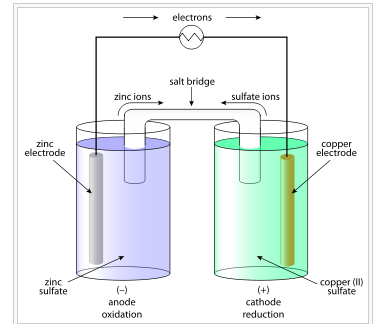
- The Galvanic cell construct the pattern of Daniel cell by taking combinations of different half cells . Each half cell consists of a metallic electrode dipped into an electrolyte .
- The two half cells are connected by a metallic wire through a voltmeter and a switch externally .
- The electrolytes of the two half cells are connected internally through a Salt Bridge . A Salt Bridge is a U – shaped containing KOH or other electrolyte solidified by agar -agar gel . It is used to make electrical connection of both the electrolytes of the cell . It is used to maintain in electrical neutrality of charges in both the beakers .
- Sometimes both the electrodes dip in the same electrolyte solution and in such cases we don’t require a Salt Bridge .
- At each electrode – electrolyte interfare there is a tendency of metal ions from the solution to deposit on the metal electrode trying to make it positively charged . At the same time metal atoms of the electrode have a tendency to go into the solution as ions and leave behind the electrons at the electrode trying to make it negatively charged .
Daniel Cell :-
Daniel Cell having electrodes of zinc and copper dipping in the solutions of their respective salt . This cell converts the chemical energy liberated during the Redox reaction
Zn ( s ) + Cu²⁺ ( aq ) + Cu ( s )
This reaction is a combination of two half reactions , whose addition gives the overall cell reaction –
1 . Cu²⁺ + 2e⁻ → Cu ( s ) , Reduction half reaction
2 . Zn ( s ) → Zn²⁺ + 2e⁻ , Oxidation half reaction .
These reactions occur in two different portions of the Daniel cell . The reduction half reaction occurs on the Copper electrode while the oxidation half reaction occurs on the Zinc electrode . These two portions of the Cell are also called half Cells or redox couples .
Electrode potential :-
A potential difference develops between the electrode and the electrolytes which is called electrode potential . When the concentrations of all the species involved in half – cell is unity then the electrode potential is known as standard electrode potential .
- The potential difference between two electrodes of a Galvanic Cell is called the Cell potential and is measured in Volt .
It is also called the electromotive force ( emf ) of the Cell when no current is drawn through the Cell .
- The emf of the Cell is positive and is given by the potential of the half – Cell on the right hand side minus the potential of the half – Cell on the left hand side .
I . e . Ecell = Eright – Eleft
Example – Cell reaction
Cu ( s ) + 2Ag⁺ ( aq ) → Cu²⁺ + 2Ag ( s )
Half Cell reactions
2Ag+ ( aq ) + 2e⁻ → 2Ag ( s ) , Reduction on cathode .
Cu ( s ) → Cu²⁺ ( aq ) + 2e⁻ , Oxidation on anode .
- Silver electrode acts as a cathode and Copper electrode acts as an anode . The Cell can be represented as –
Cu ( s ) | Cu²⁺ ( aq ) | | Ag⁺ ( aq ) | Age ( s )
then the emf of the Cell
Ecell = Eright – Eleft = EAg⁺ / Ag – ECu²⁺ / Cu
Measurement of Electrode potential :-

According to convention a half – Cell called a standard hydrogen electrode represented by Pt ( s )| H₂ ( g ) | H⁺ ( aq ) is assigned a zero potential at all temperature corresponding to the reaction
H⁺ ( aq ) + e⁻ → 1 / 2 H₂ ( g )
- The standard hydrogen electrode consists of a Platinum electrode coated with Platinum black .The electrode is dipped in an acidic solution and pure hydrogen gas is bubbled through it . The concentration of both the reduced and oxidised forms of hydrogen is maintained at Unity . The pressure of hydrogen gas is 1 bar and concentration of hydrogen ion in the solution is 1 molar .
- If the concentrations of the oxidised and reduced forms of the species in the right of Cell are unity then the Cell potential is equal to standard electrode potential , E₁ of the given half Cell
E = E₁ – E₂
As E₂ for standard hydrogen electrode is zero .
E = E₁ – 0 = E₁
The measured emf of the Cell :-
Pt ( s ) | H₂ ( g , 1 bar ) | H⁺ ( aq , 1 M ) || Cu²⁺ ( aq , 1 M ) | Cu is 0·34 and it is also the value for the Standard hydrogen electrode of the half cell corresponding to the reaction
Cu²⁺ ( aq , 1 M ) + 2e⁻ → Cu ( s )
Electrochemical cell and Gibbs energy of the reaction :-
Electrical work done in one second is equal to electrical potential multiplied by total charge passed . If we want to obtain maximum work from a Galvanic cell then charge has to be passed reversibly . The reversible work done by a Galvanic cell is equal to decrease in its Gibbs energy and therefore if the emf of the cell is E and nF is the amount of charge passed and ∆G is the Gibbs energy of the reaction then
∆G = – nF Ecell
The value of ∆G is depends on n . Ecell is an intensive parameter but ∆G is extensive thermodynamic property .
If we write the reaction
Zn ( s ) + Cu²⁺ ( aq ) → Zn²⁺ ( aq ) + Cu ( s )
∆G = – 2F Ecell
When we write the reaction
2 Zn ( s ) + 2 Cu²⁺ ( aq ) → 2 Zn²⁺ ( aq ) + 2Cu ( s )
∆G = – 4F Ecell
If the concentration of all the reacting species is unity , then
Ecell = E’cell
∆G = – nF E’cell
Thus , from the measurement of E’cell we can obtain an important thermodynamic quantity ∆G , standard Gibbs energy of the reaction .
From the latter we can calculate equilibrium constant by the equation
∆G = – R T InK
Battery or Cells :-
Batteries are a collection of one or more cells whose chemical reaction create of flow of electrons in a circuit .
All batteries are made up of three basic components :- an anode ( the negative side ) , a cathode ( the positive side ) and some kind of electrolytes ( substances that chemically with the anode and cathode )
There are mainly two types of batteries :-
1 .Primary batteries and
2 . Secondary batteries .
Primary batteries :-
In the primary batteries , the reaction occurs only once and after use over a period of time battery becomes dead and cannot be reused again .
Examples – Dry cell ( Leclanche cell ) , Galvanic Cell or Voltaic Cell .

- It was first discovered by Leclanche in 1868 it is used commonly in transistors and clocks .
- The cell consists of a Zinc container that also acts as anode and the cathode is a a carbon ( graphite ) rode surrounded by powdered magnese dioxide and carbon .
The space between the electrodes is filled by a moist paste of Ammonium Chloride and Zinc Chloride .
- The electrode reactions are complex but they can be written as
Anode : Zn ( s ) → Zn²⁺ + 2e⁻
Cathode : MnO₂ + NH₄⁺ + e⁻ → MnO ( OH ) + NH₃
In the reaction at Cathode magnese is reduced from the +4 Oxidation state to +3 state . Ammonia produced in the reaction forms a complex with Zn²⁺ to give [ Zn ( NH₃ )₄ ]²⁺
The Cell has a potential of nearly 1.5 volt .
Secondary batteries :-
Secondary batteries after use can be recharged by passing current through it in the opposite direction so that it can be used again . A good secondary Cell can undergo a large number of discharging and charging cycles .
Examples – Lead storage battery , Nickel Cadmium Cell .

It consists of a lead anode and a grid of lead packed with lead oxide ( PbO₂ ) as cathode . A 38% solution of Sulphuric acid is used as electrolytes .
The cell reactions when the battery is in use are given as
Anode : Pb ( s ) + SO₄²⁻ ( aq ) → PbSO₄ ( s ) 2e⁻
Cathode : PbSO₂ ( s ) SO₄²⁻ ( aq ) + 4 H⁺ ( aq ) + 2e⁻ → PbSO₄ ( s ) + 2 H₂O ( l )
i . e . overall Cell reaction consisting of cathode and anode reaction is
Pb ( s ) + PbO₂ ( s ) + 2 H₂SO₄ ( aq ) → 2 PbSO₄ ( s ) + 2 H₂O ( l )
On charging the battery the reaction is reversed and PbSO₄ ( s ) on anode and Cathode is converted into Pb and PbO₂ respectively .