Semiconductors :-
Semiconductors are those material which has the conduction properties in between conductor and insulator. It means semiconductor do not allow the free electron to flow as conductor.
Examples :- Silicon (Si), Boron (B), Carbon (C), Germanium (Ge) etc.
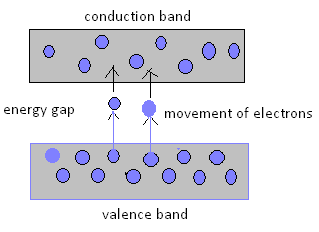
The reason for such type of conductor is the small gap between the valence band and conduction band.
Semiconductors have comparatively less free electron than the conductor.
- The conductivity of a semiconductor increases while the resistivity decreases with increase in temperature.
Types of Semiconductor :-
Two types –
1. Intrinsic semiconductor.
2. Extrinsic semiconductor.
Intrinsic semiconductor :-
Semiconductors which are chemically pure, meaning free of impurities are called intrinsic semiconductors.
The most common intrinsic semiconductors are silicon and germanium, which belong to group 4 of the periodic table. They have four valence electron and are responsible for the conduction properties of the semiconductors.
- The intrinsic semiconductors act as insulators at 0 Kelvin temperature. However at room temperature, the thermal energy may cause a few of the covalent bonds to break, thus generating the free electrons.
- The electron thus generated get existed and move into the conduction band from the valence band, overcoming the energy barrier. During this process, each electron leaves behind a hole in the valence band. The electrons and holes create in this way are called intrinsic charge carriers and are responsible for the conductive properties exhibited by the intrinsic semiconductor materials.

- In intrinsic semiconductor the number of free electrons, nₑ is equal to the number of holes nₕ
i. e. nₑ = nₕ = nᵢ
Where nᵢ is called intrinsic carrier concentration.
Under the action of an electric field, these holes move towards negative potential giving the hole current Iₕ.
The total current, I is thus the sum of the electron current Iₑ
and the hole current Iₕ
i. e. I = Iₑ + Iₕ
Extrinsic semiconductor :-
When a small amount of a suitable impurity is added to pure semiconductor, the conductivity of the semiconductor is increased. Such materials are known as extrinsic semiconductor.
- The impurity atoms are called dopants, there are two types of dopants used in doping the tetravalent Si and Ge :-
(a) pentavalent like Arsenic (As), Antimony (Sb), Phosphorus (P) etc
((b) Trivalent like Indium (In), Boron (B), Aluminium (Al) etc
Types of extrinsic semiconductor :-
- n-type semiconductor.
- p-type semiconductor.
n-type Semiconductor :-
When a small amount of impurity is added to a pure semiconductor providing a large number of free electrons in it. The extensive semiconductor thus formed is known as n-type semiconductor.

- The conduction in the n-type semiconductor is because of the free electrons denoted by the pentavalent impurity atoms. These electrons are the excess free electrons with regards to the number of free electrons required to fill the covalent bonds in the semiconductors.
- An ionisation energy required to set this free electron is very small and even at room temperature it will be free to move in the lattice of the semiconductor.
Examples :- the energy required approx 0.01 electron volt for Ge, 0.05 electron volt for Si to separates this electron from its atom.
- In an extrinsic semiconductor with pentavalent impurity, electrons become the majority carriers and holes the minority carriers. These are therefore known as n-type semiconductors.
p-type Semiconductor:-
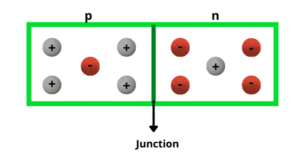
A p-type semiconductor has more holes than electrons. This allows the current to flow along the material from hole to hole but only in one direction.
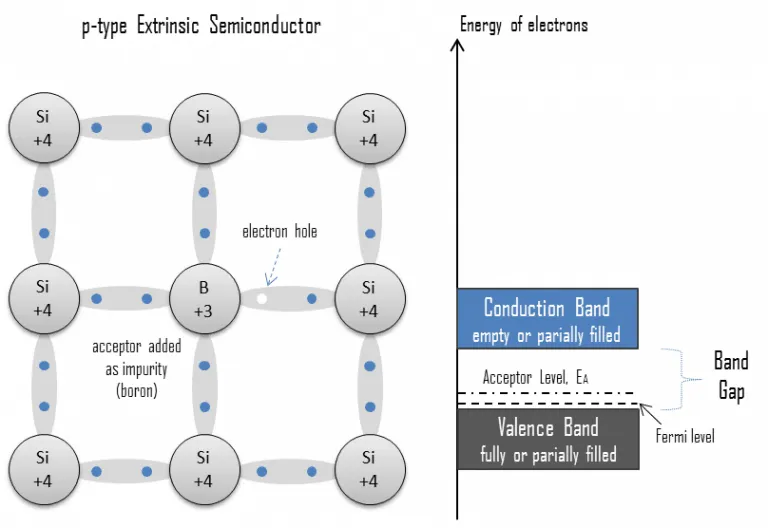
- When Si or Ge is doped with a trivalent impurity like aluminium, Boron, Indium etc. The dopant has one valence electron less than silicon or germanium and therefore, this atom can form covalent bonds with neighbouring three silicon atom but does not have any electron to offer to the fourth silicon atom. So the bond between the fourth neighbouring and the trivalent atom has a vacancy or hole.
Since the neighbouring Si atom in the lattice wants and electron in place of a whole and electron in the outer orbit of an atom in the never would mein jump to feel this vecancy leaving a vacancy for hole at own site does the whole is available for conduction